Abstract
The Hedgehog (Hh) signalling pathway plays a critical role in regulating liver lipid metabolism and related diseases. However, the underlying mechanisms are poorly understood. Here, we show that the Hh signalling pathway induces a previously undefined long non-coding RNA (Hilnc, Hedgehog signalling-induced long non-coding RNA), which controls hepatic lipid metabolism. Mutation of the Gli-binding sites in the Hilnc promoter region (HilncBM/BM) decreases the expression of Hilnc in vitro and in vivo. HilncBM/BM and Hilnc-knockout mice are resistant to diet-induced obesity and hepatic steatosis through attenuation of the peroxisome proliferator-activated receptor signalling pathway, as Hilnc directly interacts with IGF2BP2 to enhance Pparγ mRNA stability. Furthermore, we identify a potential functional human homologue of Hilnc, h-Hilnc, which has a similar function in regulating cellular lipid metabolism. These findings uncover a critical role of the Hh-Hilnc–IGF2BP2 signalling axis in lipid metabolism and suggest a potential therapeutic target for the treatment of diet-induced hepatic steatosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
RNA-seq data can be viewed in NODE (https://www.biosino.org/node/) under accession OEP001927. Biological pathways (KEGG) were analysed using DAVID (v6.8). Source data are provided with this paper. Other data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Rubin, L. L. & de Sauvage, F. J. Targeting the Hedgehog pathway in cancer. Nat. Rev. Drug Discov. 5, 1026–1033 (2006).
Ingham, P. W. & McMahon, A. P. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15, 3059–3087 (2001).
Kinzler, K. W. & Vogelstein, B. The GLI gene encodes a nuclear protein which binds specific sequences in the human genome. 10, 634–642 (1990).
Hallikas, O. et al. Genome-wide prediction of mammalian enhancers based on analysis of transcription-factor binding affinity. Cell 124, 47–59 (2006).
Pospisilik, J. A. et al. Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell 140, 148–160 (2010).
Suh, J. M. et al. Hedgehog signaling plays a conserved role in inhibiting fat formation. Cell Metab. 3, 25–34 (2006).
Claussnitzer, M. et al. FTO obesity variant circuitry and adipocyte browning in humans. N. Engl. J. Med. 373, 895–907 (2015).
Teperino, R. et al. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell 151, 414–426 (2012).
El-Agroudy, N. N., El-Naga, R. N., El-Razeq, R. A. & El-Demerdash, E. Forskolin, a hedgehog signalling inhibitor, attenuates carbon tetrachloride-induced liver fibrosis in rats. Br. J. Pharmacol. 173, 3248–3260 (2016).
Guillen-Sacoto, M. J. et al. Human germline hedgehog pathway mutations predispose to fatty liver. J. Hepatol. 67, 809–817 (2017).
Gao, L., Zhang, Z., Zhang, P., Yu, M. & Yang, T. Role of canonical Hedgehog signaling pathway in liver. Int. J. Biol. Sci. 14, 1636–1644 (2018).
Marbach-Breitruck, E. et al. Tick-tock Hedgehog-mutual crosstalk with liver circadian clock promotes liver steatosis. J. Hepatol. 70, 1192–1202 (2019).
Guy, C. D. et al. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human non-alcoholic fatty liver disease. Hepatology 55, 1711–1721 (2012).
Swiderska-Syn, M. et al. Hedgehog pathway and pediatric non-alcoholic fatty liver disease. Hepatology 57, 1814–1825 (2013).
Kwon, H. et al. Inhibition of Hedgehog signaling ameliorates hepatic inflammation in mice with non-alcoholic fatty liver disease. Hepatology 63, 1155–1169 (2016).
Choi, S. S., Omenetti, A., Syn, W. K. & Diehl, A. M. The role of Hedgehog signaling in fibrogenic liver repair. Int. J. Biochem. Cell Biol. 43, 238–244 (2011).
Matz-Soja, M. et al. Hedgehog signaling is a potent regulator of liver lipid metabolism and reveals a GLI-code associated with steatosis. Elife 5, e13308 (2016).
Vokes, S. A., Ji, H., Wong, W. H. & McMahon, A. P. A genome-scale analysis of the cis-regulatory circuitry underlying sonic Hedgehog-mediated patterning of the mammalian limb. Genes Dev. 22, 2651–2663 (2008).
Vokes, S. A. et al. Genomic characterization of Gli-activator targets in sonic Hedgehog-mediated neural patterning. Development 134, 1977–1989 (2007).
Lee, E. Y. et al. Hedgehog pathway-regulated gene networks in cerebellum development and tumorigenesis. Proc. Natl Acad. Sci. USA 107, 9736–9741 (2010).
Morris, K. V. & Mattick, J. S. The rise of regulatory RNA. Nat. Rev. Genet. 15, 423–437 (2014).
Huarte, M. The emerging role of lncRNAs in cancer. Nat. Med. 21, 1253–1261 (2015).
Prasanth, K. V. & Spector, D. L. Eukaryotic regulatory RNAs: an answer to the ‘genome complexity’ conundrum. Genes Dev. 21, 11–42 (2007).
Lee, J. T. Epigenetic regulation by long noncoding RNAs. Science 338, 1435–1439 (2012).
Geisler, S. & Coller, J. RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat. Rev. Mol. Cell Biol. 14, 699–712 (2013).
Quinn, J. J. & Chang, H. Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 17, 47–62 (2016).
Mercer, T. R., Dinger, M. E. & Mattick, J. S. Long non-coding RNAs: insights into functions. Nat. Rev. Genet. 10, 155–159 (2009).
Ponting, C. P., Oliver, P. L. & Reik, W. Evolution and functions of long non-coding RNAs. Cell 136, 629–641 (2009).
Rinn, J. L. & Chang, H. Y. Genome regulation by long non-coding RNAs. Annu. Rev. Biochem. 81, 145–166 (2012).
Chen, L. L. Linking long non-coding RNA localization and function. Trends Biochem. Sci. 41, 761–772 (2016).
Chan, L. H. et al. Hedgehog signaling induces osteosarcoma development through Yap1 and H19 overexpression. Oncogene 33, 4857–4866 (2014).
Xing, Z. et al. lncRNA directs cooperative epigenetic regulation downstream of chemokine signals. Cell 159, 1110–1125 (2014).
Zhou, M. et al. LncRNA-Hh strengthen cancer stem cells generation in twist-positive breast cancer via activation of Hedgehog signaling pathway. Stem Cells 34, 55–66 (2016).
Del Rosario, B. C. et al. Genetic intersection of Tsix and Hedgehog signaling during the initiation of X-chromosome inactivation. Dev. Cell 43, 359–371 (2017).
Wu, J. et al. The long non-coding RNA lncHDAC2 drives the self-renewal of liver cancer stem cells via activation of Hedgehog signaling. J. Hepatol. 70, 918–929 (2019).
Lin, B. J. et al. LncRNA-XIST promotes dermal papilla induced hair follicle regeneration by targeting miR-424 to activate hedgehog signaling. Cell. Signal. 72, 109623 (2020).
Zhou, H. et al. LncRNA-cCSC1 modulates cancer stem cell properties in colorectal cancer via activation of the Hedgehog signaling pathway. J. Cell. Biochem. 121, 2510–2524 (2020).
Lauth, M., Bergström, Å., Shimokawa, T. & Toftgård, R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc. Natl Acad. Sci. USA 104, 8455–8460 (2007).
Ran, F. A. et al. Genome engineering using the CRISPR–Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Yao, Q., Liu, J., Xiao, L. & Wang, N. Sonic Hedgehog signaling instigates high-fat diet-induced insulin resistance by targeting PPARγ stability. J. Biol. Chem. 294, 3284–3293 (2019).
Venteclef, N., Jakobsson, T., Steffensen, K. R. & Treuter, E. Metabolic nuclear receptor signaling and the inflammatory acute-phase response. Trends Endocrinol. Metab. 22, 333–343 (2011).
Semple, R. K., Chatterjee, V. K. & O’Rahilly, S. PPARγ and human metabolic disease. J. Clin. Invest. 116, 581–589 (2006).
Vidal-Puig, A. et al. Regulation of PPARγ gene expression by nutrition and obesity in rodents. J. Clin. Invest. 97, 2553–2561 (1996).
Matsusue, K. et al. Liver-specific disruption of PPARγ in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J. Clin. Invest. 111, 737–747 (2003).
Lee, Y. J. et al. Nuclear receptor PPARγ-regulated monoacylglycerol O-acyltransferase 1 (MGAT1) expression is responsible for the lipid accumulation in diet-induced hepatic steatosis. Proc. Natl Acad. Sci. USA 109, 13656–13661 (2012).
Hafner, M. et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 141, 129–141 (2010).
Regue, L., Minichiello, L., Avruch, J. & Dai, N. Liver-specific deletion of IGF2 mRNA binding protein-2/IMP2 reduces hepatic fatty acid oxidation and increases hepatic triglyceride accumulation. J. Biol. Chem. 294, 11944–11951 (2019).
Fornes, O. et al. JASPAR 2020: update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 48, D87–D92 (2020).
Li, Z. et al. An HMGA2–IGF2BP2 axis regulates myoblast proliferation and myogenesis. Dev. Cell 23, 1176–1188 (2012).
Christiansen, J., Kolte, A. M., Hansen, T. & Nielsen, F. C. IGF2 mRNA-binding protein 2: biological function and putative role in type 2 diabetes. J. Mol. Endocrinol. 43, 187–195 (2009).
Dai, N. et al. IGF2BP2/IMP2-deficient mice resist obesity through enhanced translation of Ucp1 mRNA and other mRNAs encoding mitochondrial proteins. Cell Metab. 21, 609–621 (2015).
Tybl, E. et al. Overexpression of the IGF2-mRNA-binding protein p62 in transgenic mice induces a steatotic phenotype. J. Hepatol. 54, 994–1001 (2011).
Schmidt-Heck, W. et al. Fuzzy modeling reveals a dynamic self-sustaining network of the GLI transcription factors controlling important metabolic regulators in adult mouse hepatocytes. Mol. Biosyst. 11, 2190–2197 (2015).
Ulitsky, I. & Bartel, D. P. lincRNAs: genomics, evolution, and mechanisms. Cell 154, 26–46 (2013).
Jiang, R. et al. Transcriptome profiling of lncRNA related to fat tissues of Qinchuan cattle. Gene 742, 144587 (2020).
Tian, K. et al. DNA and RNA editing without sequence limitation using the flap endonuclease 1 guided by hairpin DNA probes. Nucleic Acids Res. 48, e117 (2020).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Kong, L. et al. CPC: assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 35, W345–W349 (2007).
Wang, L. et al. CPAT: Coding-Potential Assessment Tool using an alignment-free logistic regression model. Nucleic Acids Res. 41, e74 (2013).
Yang, H., Wang, H. & Jaenisch, R. Generating genetically modified mice using CRISPR–Cas-mediated genome engineering. Nat. Protoc. 9, 1956–1968 (2014).
Li, W. C., Ralphs, K. L. & Tosh, D. Isolation and culture of adult mouse hepatocytes. Methods Mol. Biol. 633, 185–196 (2010).
Kawaguchi, T. et al. SWI/SNF chromatin-remodeling complexes function in non-coding RNA-dependent assembly of nuclear bodies. Proc. Natl Acad. Sci. USA 112, 4304–4309 (2015).
Harada, N. et al. Hepatic de novo lipogenesis is present in liver-specific ACC1-deficient mice. Mol. Cell Biol. 27, 1881–1888 (2007).
Acknowledgements
We thank the animal facility for animal husbandry, and technical supports from Core Facility for Cell Biology and the Genome Tagging Project Center, at CEMC. We acknowledge W. Yang for the providing reagents and helpful comments. This study was supported by grants from the National Key Research and Development Program of China (2020YFA0509000 and 2017YFA0503600 to Y.Z. and 2017YFA0505500 to D.G.), the National Natural Science Foundation of China (32130025 and 31630047 to Y.Z., 81772723 and 81830054 to D.G., 81874201 to M.B.L. and 31801184 to J.Y.P.), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16020905 to D.G.), the Basic Frontier Science Research Program of the Chinese Academy of Sciences (ZDBS-LY-SM015 to D.G.), the CAS-VPST Silk Road Science Fund 2019 (GJHZ201968 to D.G.) and the Science and Technology Commission of Shanghai Municipality (20Y11908300 to M.B.L.).
Author information
Authors and Affiliations
Contributions
Y.Z. conceived and designed the experimental approach. Y.A.J. performed most of the experiments. J.Y.P. contributed to the mouse experiments and statistical analysis. J.W.S., M.J., J.W., L.Y.M., Y.A.W. and J.H. helped the experiments and provided technical support. M.B.L., Z.Z., H.L.W. and D.G. helped to design experiments and provided useful discussion. Y.A.J. and Y.Z. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of Hilnc.
a, One TSS was identified with 5’ cap adapter using 5’ RACE primer, and two transcriptional ending sites were identified with 3’ poly (A) adapter using 3’ RACE primer in RACE assay. The upper part showed the two isoforms of Hilnc. b, Relative expression of two isoforms of Hilnc in NIH-3T3 cells was measured by qPCR (n = 3 biologically independent samples). c, A description of Hilnc’s locus. d, The full sequence of two isoforms of Hilnc. e, Hilnc accumulated in the cytoplasm. Total NIH-3T3 cell lysate was separated into cytoplasmic and nuclear fractions, and analyzed by western blot and qPCR (n = 3 biologically independent samples). For western blot, β-Tubulin was employed as a control for cytoplasm and Histone H3 for nucleolus. For qPCR, Gapdh was employed as a control for cytoplasm and U6 for nucleolus. f, A luciferase reporter driven by the WT Hilnc promoter was transfected with different concentrations of GLI1 (n = 3 biologically independent samples). The P value was calculated by two-tailed unpaired t-test. Data are means ± SEM.
Extended Data Fig. 2 The expression of Hilnc, Gli1, Ptch1 in the different organs of mice fed a NCD or HFD.
a,b,c, Total RNA was extracted from the muscle (a), WAT (b) or BAT (c) of 22-week-old male mice fed a NCD or HFD for 16 weeks (n = 3 biologically independent samples). Relative expression levels were normalized to the 18 s RNA level. The P value was calculated by two-tailed unpaired t-test. Data are means ± SEM.
Extended Data Fig. 3 Generation and phenotype of Hilnc-/- and HilncBM/BM mice.
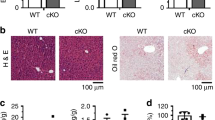
a, PCR analysis of 4-week-old mice revealed the presence of Hilnc-/- and HilncBM/BM mice as expected. b,c,d,e, Relative expression of Hilnc in the MEF (b), muscle (c), WAT (d) or BAT (e) of Hilnc-/- and HilncBM/BM mice (n = 3 biologically independent samples). f,g, Representative images (f) and body weights (g, n = 4 biologically independent samples) were monitored in 8-week-old WT, Hilnc-/- or HilncBM/BM male mice. h, Glucose tolerance tests of 10-week-old WT and HilncBM/BM male mice fed a NCD (n = 4 biologically independent animals). i, Insulin tolerance tests of 10-week-old WT and HilncBM/BM male mice fed a NCD (n = 4 biologically independent animals). j, Growth curves of WT and Hilnc-/- mice on a NCD and HFD, respectively (n = 5 biologically independent animals). The P values were 0.0044, 0.0001, 0.0050, 0.0052, 0.0082, 0.4215, 0.0067, 0.0042, 0.0051 and 0.0050 (left to right). k, Representative images of 22-week-old WT and Hilnc-/- male mice fed a HFD for 16 weeks. l, Representative images of abdominal WAT (upper) and liver (lower) from 22-week-old WT or Hilnc-/- male mice fed a HFD for 16 weeks. m, H&E and oil Red O staining of liver sections from WT and Hilnc-/- mice fed a HFD; scale bar, 50 μm. The P value was calculated by two-tailed unpaired t-test. Data are means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
Extended Data Fig. 4 Food intake, fecal fat, physical activity counts, respiratory quotient and energy expenditure in WT and HilncBM/BM mice.
a,b,c, Food intake (a), physical activity per 24 hours in X-Y direction (b), respiratory quotient (RER) (c) of male WT and HilncBM/BM mice at 14 weeks of age on a NCD (left, n = 4 biologically independent animals) and HFD (right, n = 6 biologically independent animals). d,e, Fecal weight (d), and percent fecal weight as TG (e) on a HFD (n = 6 biologically independent animals). f, Energy expenditure (EE) of WT and HilncBM/BM mice at 14 weeks of age on a NCD (left, n = 4 biologically independent animals) and HFD (right, n = 6 biologically independent animals). The P value was calculated by two-tailed unpaired t-test. Data are means ± SEM.
Extended Data Fig. 5 The overall metabolic characteristics of mice with liver-specific Hilnc manipulation.
a,b,c, Body weight (a), energy expenditure (b), and glucose tolerance test (c) of Hilnc knockdown mice and WT maintained on HFD for 10 weeks (n = 5 biologically independent animals). d,e,f, Body weight (d), energy expenditure (e), and glucose tolerance test (f) of Hilnc-/- (O/E-Hilnc) mice and Hilnc-/- (AAV8-Control) mice maintained on HFD for 10 weeks (n = 5 biologically independent animals). The data represent the mean ± SEM. Two-tailed unpaired t test was used to compare the difference between groups.
Extended Data Fig. 6 The gene expression level in the livers of WT and HilncBM/BM mice fed a NCD or HFD.
a, Volcano plot showing the changed genes in the livers of 22-week-old HilncBM/BM mice fed a NCD for 16 weeks compared with WT livers. b, KEGG pathway enrichment analysis of transcripts differentially expressed between livers isolated from WT and HilncBM/BM mice fed a NCD. c, Upregulated KEGG pathway enrichment analysis of transcripts differentially expressed between livers isolated from WT and HilncBM/BM mice fed a HFD. d, Heat map showing the changed genes that were enriched in different upregulated KEGG pathways. The heatmap is draw based on normalized expression levels. e, Western bolt showed the protein level of PPARγ in the livers of WT and HilncBM/BM mice fed a NCD.
Extended Data Fig. 7 Hilnc has no cis activity.
a, Locus of Hilnc (red) and its nearby coding genes (blue), Traf3ip2 and Fyn, on chromosome 10. b, The mRNA (left, n = 3 biologically independent samples) and protein level (right, n = 2 biologically independent samples) of Traf3ip2 and Fyn in the livers of WT and Hilnc-/- mice. c, The mRNA (left, n = 3 biologically independent samples) and protein levels (right, n = 2 biologically independent samples) of Traf3ip2 and Fyn in the livers of WT and HilncBM/BM mice. The P value was calculated by two-tailed unpaired t-test. Data are means ± SEM.
Extended Data Fig. 8 Identification of potential human homolog of Hilnc.
a, The locus of Hilnc on chromosome 10 in mouse genome. b, The relative locus of Hilnc in human genome. c, One TSS was identified using 5’ RACE primer, and one transcriptional ending sites was identified using 3’ RACE primer in RACE assay. d,e, The locus of ENST00000417084.1 (h-Hilnc) in human genome and the relative locus of h-Hilnc in mouse genome. f, The whole sequence of h-Hilnc. g, The conservation information of h-Hilnc in NONCODE.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 5–7
Supplementary Table 2
The DEGs in the livers of WT and HilncBM/BM mice fed a HFD.
Supplementary Table 3
The sequencing data of genes and lncRNAs in the immunoprecipitation samples.
Supplementary Table 4
The differentially expressed lncRNAs in the LO2 cells treated with and without OA.
Source data
Source Data Fig. 6
Unprocessed western blots.
Source Data Fig. 7
Unprocessed western blots and gels.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Rights and permissions
About this article
Cite this article
Jiang, Y., Peng, J., Song, J. et al. Loss of Hilnc prevents diet-induced hepatic steatosis through binding of IGF2BP2. Nat Metab 3, 1569–1584 (2021). https://doi.org/10.1038/s42255-021-00488-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-021-00488-3
This article is cited by
-
The m6A reader IGF2BP2 regulates glycolytic metabolism and mediates histone lactylation to enhance hepatic stellate cell activation and liver fibrosis
Cell Death & Disease (2024)
-
Sonic hedgehog-heat shock protein 90β axis promotes the development of nonalcoholic steatohepatitis in mice
Nature Communications (2024)
-
Epigenetic regulation in metabolic diseases: mechanisms and advances in clinical study
Signal Transduction and Targeted Therapy (2023)
-
Metformin can mitigate skeletal dysplasia caused by Pck2 deficiency
International Journal of Oral Science (2022)