Abstract
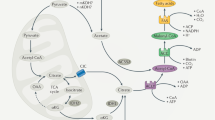
Carbohydrate can be converted into fat by de novo lipogenesis, a process upregulated in fatty liver disease. Chemically, de novo lipogenesis involves polymerization and reduction of acetyl-CoA, using NADPH as the electron donor. The feedstocks used to generate acetyl-CoA and NADPH in lipogenic tissues remain, however, unclear. Here we show using stable isotope tracing in mice that de novo lipogenesis in adipose is supported by glucose and its catabolism via the pentose phosphate pathway to make NADPH. The liver, in contrast, derives acetyl-CoA for lipogenesis from acetate and lactate, and NADPH from folate-mediated serine catabolism. Such NADPH generation involves the cytosolic serine pathway in liver running in the opposite direction to that observed in most tissues and tumours, with NADPH made by the SHMT1–MTHFD1–ALDH1L1 reaction sequence. SHMT inhibition decreases hepatic lipogenesis. Thus, liver folate metabolism is distinctively wired to support cytosolic NADPH production and lipogenesis. More generally, while the same enzymes are involved in fat synthesis in liver and adipose, different substrates are used, opening the door to tissue-specific pharmacological interventions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Raw data are provided in source data files. Source data are provided with this paper.
Code availability
El-MAVEN v.12 and natural abundance correction software accucor are available online on GitHub.
References
Lehninger A. L., Nelson D. L. & Cox M. M. Lehninger Principles of Biochemistry 6th edn (W. H. Freeman, 2013).
Yu, Y., Clippinger, A. J. & Alwine, J. C. Viral effects on metabolism: changes in glucose and glutamine utilization during human cytomegalovirus infection. Trends Microbiol. 19, 360–367 (2011).
Heaton, N. S. et al. Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis. Proc. Natl Acad. Sci. USA 107, 17345–17350 (2010).
Singh, A. et al. De novo lipogenesis represents a therapeutic target in mutant Kras non-small cell lung cancer. FASEB J. 32, 7018–7027 (2018).
Stoiber, K. et al. Targeting de novo lipogenesis as a novel approach in anti-cancer therapy. Br. J. Cancer 118, 43–51 (2018).
Lambert, J. E., Ramos-Roman, M. A., Browning, J. D. & Parks, E. J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 146, 726–735 (2014).
Bence, K. K. & Birnbaum, M. J. Metabolic drivers of non-alcoholic fatty liver disease. Mol. Metab. 50, 101143 (2020).
Jang, C. et al. The small intestine shields the liver from fructose-induced steatosis. Nat. Metab. 2, 586–593 (2020).
Goldberg, R. P. & Brunengraber, H. Contributions of cytosolic and mitochondrial acetyl-CoA syntheses to the activation of lipogenic acetate in rat liver. Adv. Exp. Med Biol. 132, 413–418 (1980).
Hellerstein, M. K., Wu, K., Kaempfer, S., Kletke, C. & Shackleton, C. H. Sampling the lipogenic hepatic acetyl-CoA pool in vivo in the rat. Comparison of xenobiotic probe to values predicted from isotopomeric distribution in circulating lipids and measurement of lipogenesis and acetyl-CoA dilution. J. Biol. Chem. 266, 10912–10919 (1991).
Chen, L. et al. NADPH production by the oxidative pentose-phosphate pathway supports folate metabolism. Nat. Metab. 1, 404–415 (2019).
Ghergurovich, J. M. et al. Glucose-6-phosphate dehydrogenase is not essential for K-ras-driven tumor growth or metastasis. Cancer Res. 80, 3820–3829 (2020).
Yoshida, A. Hemolytic anemia and G6PD deficiency. Science 179, 532–537 (1973).
Fan, J. et al. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 510, 298–302 (2014).
Liu, L. et al. Malic enzyme tracers reveal hypoxia-induced switch in adipocyte NADPH pathway usage. Nat. Chem. Biol. 12, 345–352 (2016).
Simopoulos, A. P. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 70, 560S–569S (1999).
Kingsbury, K. J., Paul, S., Crossley, A. & Morgan, D. M. The fatty acid composition of human depot fat. Biochem. J. 78, 541–550 (1961).
Wadke, M., Brunengraber, H., Lowenstein, J. M., Dolhun, J. J. & Arsenault, G. P. Fatty acid synthesis by liver perfused with deuterated and tritiated water. Biochemistry 12, 2619–2624 (1973).
Jungas, R. Fatty acid synthesis in adipose tissue incubated in tritiated water. Biochemistry 7, 3708-& (1968).
Seyama, Y. et al. Identification of sources of hydrogen-atoms in fatty-acids synthesized using deuterated water and stereospecifically deuterium labeled nadph by gas-chromatographic mass-spectrometric analysis. Biomed. Mass Spectrom. 5, 357–361 (1978).
Diraison, F., Pachiaudi, C. & Beylot, M. In vivo measurement of plasma cholesterol and fatty acid synthesis with deuterated water: determination of the average number of deuterium atoms incorporated. Metabolism 45, 817–821 (1996).
Hellerstein, M. K. et al. Measurement of de novo hepatic lipogenesis in humans using stable isotopes. J. Clin. Invest. 87, 1841–1852 (1991).
McCabe, B. J. & Previs, S. F. Using isotope tracers to study metabolism: application in mouse models. Metab. Eng. 6, 25–35 (2004).
Zhang, Z., Chen, L., Liu, L., Su, X. & Rabinowitz, J. D. Chemical basis for deuterium labeling of fat and NADPH. J. Am. Chem. Soc. 139, 14368–14371 (2017).
Hellerstein, M. K. No common energy currency: de novo lipogenesis as the road less traveled. Am. J. Clin. Nutr. 74, 707–708 (2001).
Hui, S. et al. Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118 (2017).
Sanchez-Gurmaches, J. et al. Brown fat AKT2 is a cold-induced kinase that stimulates ChREBP-mediated de novo lipogenesis to optimize fuel storage and thermogenesis. Cell Metab. 27, 195–209.e6 (2018).
Vijayakumar, A. et al. Absence of carbohydrate response element binding protein in adipocytes causes systemic insulin resistance and impairs glucose transport. Cell Rep. 21, 1021–1035 (2017).
Hui, S. et al. Quantitative fluxomics of circulating metabolites. Cell Metab. 32, 676–688 (2020).
Faubert, B. et al. Lactate metabolism in human lung tumors. Cell 171, 358–371 (2017).
Gladden, L. B. Lactate metabolism: a new paradigm for the third millennium. J. Physiol. 558, 5–30 (2004).
Gladden, L. B. A lactatic perspective on metabolism. Med Sci. Sports Exerc. 40, 477–485 (2008).
Mashimo, T. et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 159, 1603–1614 (2014).
Schug, Z. T. et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell 27, 57–71 (2015).
Comerford, S. A. et al. Acetate dependence of tumors. Cell 159, 1591–1602 (2014).
Huang, Z. et al. ACSS2 promotes systemic fat storage and utilization through selective regulation of genes involved in lipid metabolism. Proc. Natl Acad. Sci. USA 115, E9499–E9506 (2018).
Zhao, S. et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature 579, 586–591 (2020).
Nelson, M. E. et al. Inhibition of hepatic lipogenesis enhances liver tumorigenesis by increasing antioxidant defence and promoting cell survival. Nat. Commun. 8, 14689 (2017).
Tasdogan, A. et al. Metabolic heterogeneity confers differences in melanoma metastatic potential. Nature 577, 115–120 (2020).
Uhlén, M. et al. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Lewis, C. A. et al. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol. Cell 55, 253–263 (2014).
Ölander, M., Wiśniewski, J. R. & Artursson, P. Cell-type-resolved proteomic analysis of the human liver. Liver Int. 40, 1770–1780 (2020).
Ding, C. et al. A cell-type-resolved liver proteome. Mol. Cell Proteom. 15, 3190–3202 (2016).
Battistuzzi, G., D’Urso, M., Toniolo, D., Persico, G. M. & Luzzatto, L. Tissue-specific levels of human glucose-6-phosphate dehydrogenase correlate with methylation of specific sites at the 3′ end of the gene. Proc. Natl Acad. Sci. USA 82, 1465–1469 (1985).
Hao, Q. et al. Transcriptome profiling of brown adipose tissue during cold exposure reveals extensive regulation of glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 308, E380–E392 (2015).
Karlsson, M. et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. https://doi.org/10.1126/sciadv.abh2169 (2021).
Metallo, C. M. et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481, 380–384 (2011).
MacParland, S. A. et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat. Commun. 9, 4383 (2018).
Davidson, S. M. et al. Environment impacts the metabolic dependencies of ras-driven non-small cell lung cancer. Cell Metab. 23, 517–528 (2016).
Piskounova, E. et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527, 186–191 (2015).
MacFarlane, A. J. et al. Cytoplasmic serine hydroxymethyltransferase regulates the metabolic partitioning of methylenetetrahydrofolate but is not essential in mice. J. Biol. Chem. 283, 25846–25853 (2008).
Ducker, G. S. et al. Reversal of cytosolic one-carbon flux compensates for loss of the mitochondrial folate pathway. Cell Metab. 24, 640–641 (2016).
Ducker, G. S. et al. Human SHMT inhibitors reveal defective glycine import as a targetable metabolic vulnerability of diffuse large B-cell lymphoma. Proc. Natl Acad. Sci. USA 114, 11404–11409 (2017).
Beaudin, A. E. et al. Shmt1 and de novo thymidylate biosynthesis underlie folate-responsive neural tube defects in mice. Am. J. Clin. Nutr. 93, 789–798 (2011).
Meiser, J. et al. Serine one-carbon catabolism with formate overflow. Sci. Adv. 2, e1601273 (2016).
Zheng, Y. et al. Mitochondrial one-carbon pathway supports cytosolic folate integrity in cancer cells. Cell 175, 1546–1560 (2018).
Appling, D. R. Compartmentation of folate-mediated one-carbon metabolism in eukaryotes. FASEB J. 5, 2645–2651 (1991).
Barlowe, C. K. & Appling, D. R. In vitro evidence for the involvement of mitochondrial folate metabolism in the supply of cytoplasmic one-carbon units. Biofactors 1, 171–176 (1988).
Softic, S. et al. Dietary sugars alter hepatic fatty acid oxidation via transcriptional and post-translational modifications of mitochondrial proteins. Cell Metab. 30, 735–753.e4 (2019).
Alwahsh, S. M. & Gebhardt, R. Dietary fructose as a risk factor for non-alcoholic fatty liver disease (NAFLD). Arch. Toxicol. 91, 1545–1563 (2017).
Hellerstein, M. K., Schwarz, J. M. & Neese, R. A. Regulation of hepatic de novo lipogenesis in humans. Annu Rev. Nutr. 16, 523–557 (1996).
Cohen, P. et al. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science 297, 240–243 (2002).
Ducker, G. S. & Rabinowitz, J. D. One-carbon metabolism in health and disease. Cell Metab. 25, 27–42 (2017).
Mullarky, E. et al. Inhibition of 3-phosphoglycerate dehydrogenase (PHGDH) by indole amides abrogates de novo serine synthesis in cancer cells. Bioorg. Med. Chem. Lett. 29, 2503–2510 (2019).
Pacold, M. E. et al. A PHGDH inhibitor reveals coordination of serine synthesis and one-carbon unit fate. Nat. Chem. Biol. 12, 452–458 (2016).
Locasale, J. W. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer 13, 572–583 (2013).
Yang, M. & Vousden, K. H. Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer 16, 650–662 (2016).
Ye, J. et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. 4, 1406–1417 (2014).
Fu, X. et al. Measurement of lipogenic flux by deuterium resolved mass spectrometry. Nat. Commun. 12, 3756 (2021).
Wallace, M. & Metallo, C. M. Tracing insights into de novo lipogenesis in liver and adipose tissues. Semin. Cell Dev. Biol. 108, 65–71 (2020).
Strawford, A., Antelo, F., Christiansen, M. & Hellerstein, M. K. Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. Am. J. Physiol. Endocrinol. Metab. 286, E577–E588 (2004).
Shi, Y. et al. -Adrenergic receptor agonist induced hepatic steatosis in mice: modeling nonalcoholic fatty liver disease in hyperadrenergic states. Am. J. Physiol. Endocrinol. Metab. 321, E90–E104 (2021).
Brunengraber, H., Kelleher, J. K. & Des Rosiers, C. Applications of mass isotopomer analysis to nutrition research. Annu Rev. Nutr. 17, 559–596 (1997).
Laugero, K. D. & Moberg, G. P. Energetic response to repeated restraint stress in rapidly growing mice. Am. J. Physiol. Endocrinol. Metab. 279, E33–E43 (2000).
Svensson, R. U. et al. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat. Med. 22, 1108–1119 (2016).
Esler, W. P. & Bence, K. K. Metabolic targets in nonalcoholic fatty liver disease. Cell Mol. Gastroenterol. Hepatol. 8, 247–267 (2019).
Kim, C. W. et al. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metab. 26, 576 (2017).
Goedeke, L. et al. Acetyl-CoA carboxylase inhibition reverses NAFLD and hepatic insulin resistance but promotes hypertriglyceridemia in rodents. Hepatology 68, 2197–2211 (2018).
Chondronikola, M. et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63, 4089–4099 (2014).
Stanford, K. I. et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Invest. 123, 215–223 (2013).
Tuli, J. S., Smith, J. A. & Morton, D. B. Stress measurements in mice after transportation. Lab Anim. 29, 132–138 (1995).
Rong, X. et al. LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell Metab. 18, 685–697 (2013).
Jang, C. et al. Metabolite exchange between mammalian organs quantified in pigs. Cell Metab. 30, 594–606 (2019).
Chen, L., Ducker, G. S., Lu, W., Teng, X. & Rabinowitz, J. D. An LC–MS chemical derivatization method for the measurement of five different one-carbon states of cellular tetrahydrofolate. Anal. Bioanal. Chem. 409, 5955–5964 (2017).
Melamud, E., Vastag, L. & Rabinowitz, J. D. Metabolomic analysis and visualization engine for LC–MS data. Anal. Chem. 82, 9818–9826 (2010).
Su, X., Lu, W. & Rabinowitz, J. D. Metabolite spectral accuracy on orbitraps. Anal. Chem. 89, 5940–5948 (2017).
Shah, V., Herath, K., Previs, S. F., Hubbard, B. K. & Roddy, T. P. Headspace analyses of acetone: a rapid method for measuring the 2H-labeling of water. Anal. Biochem. 404, 235–237 (2010).
Eipel, C., Abshagen, K. & Vollmar, B. Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J. Gastroenterol. 16, 6046–6057 (2010).
Acknowledgements
This work was supported by NIH Pioneer award 1DP1DK113643 and Pfizer. T.T. is supported by NIH fellowship 1F32DK118856. L.Y. was supported by a postdoctoral fellowship from the New Jersey Commission on Cancer Research. We thank X. Rong for input and advice. We thank S. Hui, C. Jang, L. Chen, Y. Shen, L. Wang, C. Bartman and all other Rabinowitz lab members for advice.
Author information
Authors and Affiliations
Contributions
Z.Z., T.T. and J.D.R. came up with the general approach and experimental strategy. Z.Z. and T.T. designed and performed the in vivo and in vitro experiments and data interpretation. X.X. performed folate species measurement. X.Z. performed acetate measurement. L.Y. contributed to the initial discovery of serine-mediated NADPH production in liver. G.X., G.J.T. and M.F.C. contributed to in vitro tracing experiments and GC–MS analysis. Z.Z., T.T. and J.D.R. wrote the manuscript with help from all authors.
Corresponding author
Ethics declarations
Competing interests
G.X., G.J.T. and M.F.C. are current employees of Pfizer and may be Pfizer shareholders. J.D.R. is a consultant and received research funding from Pfizer and is an adviser and stock owner in Colorado Research Partners, L.E.A.F. Pharmaceuticals, Rafael Pharmaceuticals, Barer Institute and its subsidiaries, Serien Therapeutics, Toran, Kadmon Pharmaceuticals and Agios Pharmaceuticals. J.D.R. is co-inventor of SHIN2 and related SHMT inhibitors, which have been patented by Princeton University. The remaining authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: Christoph Schmitt
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Fatty acid composition and D2O labeling across tissues and time.
(a) Fraction of tissue weight that is fat, and composition of those fatty acids in male and female mice. (b) Fat is stored in white adipose tissue, and to a lesser extent in muscle. Muscle values are calculated assuming quadriceps is representative of whole-body muscle. (c) Body weight of mice drinking 20% D2O for 4 weeks (control data are from Jackson lab website). (d) Fatty acid labeling from D2O reaches steady-state more quickly in serum than in white adipose, while different fatty acid species reach steady-state at a similar rate. (e) C18:0 and oleate (C18:1) labeling pattern across tissues after 4 weeks of D2O drinking. (f) Serum D2O enrichment measured by LC-MS and GC-MS. (g) Labeling of serum D2O increases linearly over time for the 12 h of infusion. (h) Circadian pattern of liver fat synthesis based on 6 h D2O infusions. (i) C16:0 labeling pattern across tissues after 12 h fed-state D2O infusion in male mice. (j) As is (j), for female mice. (k) C16:0 labeling in white adipose tissue, liver, and brown adipose tissue from 12 h refed D2O infusion after 24 h fast. Mean ± s.d., n = 3 of both genders for fat composition analysis; n = 4 male mice for steady-state analysis and body composition analysis; n = 4 of both genders for bodyweight measurement; n = 2 male mice for 6 h D2O infusions; n = 5 of both genders for 12 h D2O infusion; n = 5 males in each group for the fasted-refed experiments.
Extended Data Fig. 2 Circulating metabolite levels and labeling and tissue saponified fat labeling from carbon tracers and [1-2H]glucose.
(a) Circulating concentrations of unlabeled and labeled forms during the indicated tracer infusions. (b) Corresponding total concentrations. (c) Corresponding average carbon atom labeling (for the 13 C tracers) or labeled fraction (for the [1-2H]glucose tracer). (d) Tail vein serum circulating metabolite average carbon atom labeling from different infused 13C tracers (each sampled at end of 12 h infusion). (e) As in (d), from portal vein. (f) Corresponding C16:0 labeling in liver and brown adipose. (g) Glucose-6-phosphate labeling from 12 h [1-2H]glucose infusion in liver and brown adipose tissue. All data are mean ± s.d. For time-point samples, [U-13C]glucose (n = 3), lactate (n = 3), acetate (n = 3), [1-2H]glucose (n = 4), saline (n = 2); for other samples [U-13C]glucose (n = 4), lactate (n = 4), glutamine (n = 2), acetate (n = 4), alanine (n = 2) citrate (n = 2) and [1-2H]glucose (n = 4). All mice are males. For female data, see Extended Data Fig. 6.
Extended Data Fig. 3 Carbon tracing in liver slices.
(a-g). Labeling of the indicated metabolites in liver slices after 2 h or 6 h incubation in Krebs-Ringer buffer containing glucose, lactate, and acetate, with the indicated metabolite provided in [U13C]-labeled form. Data are mean ± s.d.; n = 2 mice for 2 h experiments; n = 3 mice for 6 h experiments, 2 technical replicates (independent tissue slices) from each mouse liver. All mice are males.
Extended Data Fig. 4 Hepatocytes are deficient in the oxPPP, and [2,3,3,4,4-2H]glutamine tracer works to probe malic enzyme and IDH flux in cultured hepatocytes but not in vivo.
(a) C16:0 labeling over 6 h from different tracers in primary hepatocytes. (b) NADPH active-H labeling over 2.5 h from different tracers in primary hepatocytes. (c) NADPH active-H measurement over 2.5 h from [3-2H]glucose in HepG2 cells. (d) Enzymatic activity of G6PD, 6PGD, IDH1 and ME1 in lysates from HepG2 cells and primary murine hepatocytes. (e) Hydride sources supporting de novo lipogenesis in HepG2 cells and primary hepatocytes, correcting for substrate labeling and H-D exchange between NADPH and water. (f) NADPH active hydrogen labeling from 12 h D2O infusion in liver and brown adipose. (g) The fraction of active hydrogen on NADPH that undergoes solvent exchange with water calculated from 12 h D2O infusion in liver and brown adipose. (h) Glutamine is the dominant TCA substrate in cultured primary hepatocytes. (i) Serum tracer labeling at the end of 12 h [2,3,3,4,4-2H]glutamine infusion. (j) Minimal malate and (iso)citrate labeling from [2,3,3,4,4-2H]glutamine infusion across tissues in vivo. (k) C16:0 labeling from [2,3,3,4,4-2H]glutamine infusion across tissues. (l) Minimal malate and (iso)citrate labeling from [4-2H]glucose and [U-2H]succinate in cultured primary hepatocytes. All data are mean ± s.d., n = 4 for solvent exchange measurement, n = 2 for [2,3,3,4,4-2H]glutamine infusion, all mice are males, n = 3 biological replicates for all cell culture experiments.
Extended Data Fig. 5 Serine’s hydride contribution to liver fat across 8-fold range of [2,3,3-2H]serine infusion rates.
(a) Circulating serine labeling during [2,3,3-2H]serine infusion at different rates, ranging from minimally to highly perturbative. (b) Lack of labeling in liver acetyl-CoA and related metabolites from 12 h [2,3,3-2H]serine infusion (20 nmol/min/gram body weight). (c) Circulating serine concentrations during [2,3,3-2H]serine infusion at different rates, ranging from minimally to highly perturbative (sum of unlabeled and labeled). (d) Corresponding serine labeling (average atom labeling of serine side chain hydrogens). (e) Liver serine labeling after 12 h [2,3,3-2H]serine infusion. (f) Corresponding liver and brown adipose tissue NADP(H) labeling. All data are mean ± s.d., For time point experiments n = 3 for each tracer and n = 2 for saline. For C16:0, NADPH, acetyl-CoA, and related metabolite labeling, n = 4 for 5 nmol/min/gram bodyweight and n = 6 for 10, 20 and 40 nmol/min/gram bodyweight of [2,3,3-2H]serine infusion. All mice are males.
Extended Data Fig. 6 Carbon and hydrogen inputs to lipogenesis are consistent across female and male mice.
(a) Tail vein serum circulating metabolite average carbon atom labeling from different infused 13C tracers (each after 12 h infusion) in female mice. (b) Corresponding portal vein serum circulating metabolite labeling in female mice. (c) Corresponding C16:0 labeling in liver and brown adipose in female mice. (d) Fractional enrichment of liver C16:0 in female mice following 12 h infusion of [U-13C]-labeled glucose (n = 3), lactate (n = 3), acetate (n = 3). Male data are the same as Fig. 2a. Labeling is normalized to circulating tracer enrichment. Brown adipose is fueled by systemic blood (approximated by tail vein sampling) and liver is fueled by 78% portal vein blood and 22% systemic blood. (e) As is (d), for brown adipose C16:0. Male data are the same as in Fig. 2b. (f) Carbon sources supporting de novo lipogenesis in liver and brown adipose in female and male mice, based on direct contributions to C16:0, calculated based on data in (d) and (e). Male data are the same as Fig. 2d. (g) C16:0 labeling in liver, brown adipose and serum following 12 h [1-2H]glucose infusion (125 nmol/min/gram body weight) in female mice (n = 3). (h) NADPH active-H measurement in liver and brown adipose from 12 h [1-2H]glucose infusion (125 nmol/min/gram bw)in female mice (n = 4). (i) As is (g), for 12 h [2,3,3-2H]serine infusion (20 nmol/min/gram bw). (j) As is (h), for 12 h [2,3,3-2H]serine infusion (20 nmol/min/gram bw). (k) Hydride sources supporting de novo lipogenesis in liver and brown adipose in female (left-hand bars) and male (right-hand bars) mice, correcting for substrate labeling and H-D exchange between NADPH and water. Calculated based on tracer data in (h) and (j) and D2O exchange data in Extended Data Fig. 4f. Male data are the same as in Fig. 4g. Light bars indicate contribution from 1C metabolism; dark bars indicate contribution from oxPPP. Data in f are mean ± s.e.m.; other data are mean ± s.d.
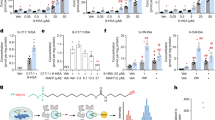
Extended Data Fig. 7 Cytosolic serine catabolism in the liver is blunted by SHMT knockout or inhibition.
(a) 5-methyl-THF labeling from 12 h [2,3,3-2H]serine (20 nmol/min/gram body weight). M + 2 labeling reflects cytosolic serine catabolism. M + 1 labeling reflects the combination of mitochondrial serine catabolism and reversible flux through MTHFD1. (b) Glucose levels after insulin tolerance test in control and whole-body ΔSHMT1 mice. (c) Serine labeling in liver from 12 h [2,3,3-2H]serine infusion (20 nmol/min/gram bw) in control, whole-body ΔSHMT1 mice, and mice treated with the dual SHMT1/2 inhibitor SHIN2 (3.33 mg/kg/h i.v. infusion). (d) C16:0 labeling in liver from 12 h [2,3,3-2H]serine infusion (20 nmol/min/gram bw). (e) As is (c), for [2H]formate infusion. (control vs. ΔSHMT1, p = 0.0010; control vs. SHIN2 treatment, p = 0.0091). (f) As is (d), for [2H]formate infusion. (g) C16:0 labeling across tissues following 12 h [2,3,3-2H]serine infusion (20 nmol/min/gram body weight) in control and ΔSHMT1 mice. Mean ± s.d. For insulin tolerance tests, n = 12 for SHMT1 knock out mice, n = 4 for littermate wild type controls. For serine and formate infusions, n = 6 for control, n = 4 for ∆SHMT1, n = 4 for SHIN2 treatment. For [2,3,3-2H]serine infusion in ∆SHMT1 mice, n = 4 for liver, bat, brain bone marrow, serum, and skin; n = 3 for other tissues; control mice data are the same as Fig. 4b. For D2O tracing experiments, n = 10 in each group. *p < 0.05; **p < 0.01; ***p < 0.005 by unpaired Mann-Whitney test. All mice are males.
Extended Data Fig. 8 Concentrations and labeling of saponified fatty acids and soluble metabolites in wild type, ΔSHMT1, and SHMT inhibitor-treated mice (3.33 mg/kg/h i.v. infusion of SHIN2 for 12 h).
(a) Saponified C16:0 labeling pattern in liver following 12 h D2O infusion to control, ΔSHMT1, and SHIN2-treated mice (SHIN2 infused simultaneous with the D2O infusion). (b) As is (a), for brown adipose. (c) Saponified C16:0 labeling pattern in liver following 12 h IP D2O to control and SHIN2-treated mice (SHIN2 infused simultaneously). (d) C16:0 lipogenesis flux in liver overnight, as measured by IP D2O injection. (control vs. SHIN2 treatment, p = 0.0047). (e) C16:0 and C18:0 concentration in liver of control, ΔSHMT1, and SHIN2-treated mice (control vs. ΔSHMT1, p = 0.48 for C16:0, p = 0.88 for C18:0; control vs. inhibitor, p = 0.48 for C16:0, p = 0.34 for C18:0). (f) As is (a), for brown adipose tissue (control vs. ΔSHMT1, p = 0.68 for C16:0, p = 0.99 for C18:0; control vs. SHIN2 treatment, p = 0.99 for C16:0, p = 0.11 for C18:1). (g) C16:0 lipogenesis flux in brown adipose tissue overnight, as measured by 12 h D2O infusion (control vs. ΔSHMT1, p = 0.32; control vs. SHIN2 treatment, p = 0.28). (h) C16:0 labeling across tissues following 12 h D2O infusion in control and ΔSHMT1 mice. (i) Serine concentration in liver and brown adipose tissue of control, ΔSHMT1, and SHIN2-treated mice (control vs. ΔSHMT1, p = 0.99 for liver, p = 0.99 for BAT; control vs. SHIN2 treatment, p = 0.34 for liver, p = 0.34 for BAT). (j) As is (i), for NADPH (control vs. ΔSHMT1, p = 0.99 for liver, p = 0.88 for BAT; control vs. SHIN2 treatment, p = 0.11 for liver, p = 0.20 for BAT). (k) As is (i), for NADP+ (control vs. ΔSHMT1, p = 0.34 for liver, p = 0.34 for BAT; control vs. SHIN2 treatment, p = 0.68 for liver, p = 0.48 for BAT). (l) As is (i), for glutathione (control vs. ΔSHMT1, p = 0.34 for liver, p = 0.68 for BAT; control vs. SHIN2 treatment, p = 0.48 for liver, p = 0.99 for BAT). (m) As is (i), for glutathione disulfide (control vs. ΔSHMT1, p = 0.34 for liver, p = 0.88 for BAT; control vs. SHIN2 treatment, p = 0.88 for liver, p = 0.14 for BAT). (n) Liver glutathione disulfide vs. reduced glutathione ratio in control, ΔSHMT1, and SHIN2-treated mice (control vs. ΔSHMT1, p = 0.99; control vs. SHIN2 treatment, p = 0.029). (o) Liver C16:0 labeling pattern after 12 h D2O infusion during ad lib feeding and in mice subjected to 24 h fasting followed by refeeding (control data are the same as Extended Data Fig. 1i; ΔSHMT1 ad lib fed data are the same as Extended Data Fig. 8h). Mean± s.d, n = 4 for control, ∆SHMT1, and SHIN2-treated mice. For refeeding experiment, n = 3 for ΔSHMT1 refed. For D2O infusion in ∆SHMT1 mice, n = 2 for liver, bat, white adipose, lung, brain, bone marrow, serum, and skin; n = 1 for other tissues; control mice data are the same as Extended Data Fig. 1i. *p < 0.05 by unpaired Mann-Whitney test. All mice are males.
Extended Data Fig. 9 Knockout of SHMT1 does not induce alternative NADPH producing enzymes.
(a) C16:0 labeling from [1-2H]glucose in liver, brown adipose tissue, and serum in control and ΔSHMT1 mice. (b) Relative mRNA expression level of Malic Enzyme 1 (ME1) and Isocitrate dehydrogenase 1 (IDH1) in liver of control, ΔSHMT1, and 12 h SHIN2 treated mice (3.33 mg/kg/h i.v. infusion) (control vs. ΔSHMT1, p = 0.48 for ME1, p = 0.88 for IDH1; control vs. inhibitor, p = 0.73 for ME1, p = 0.56 for IDH1.). (c) Relative mRNA expression level of fatty acid synthase (FASN) in liver of control, ΔSHMT1, and 12 h SHIN2 treated mice (3.33 mg/kg/h i.v. infusion) (control vs. ΔSHMT1, p = 0.48 for control vs. inhibitor, p = 0.26.) Mean± s.d. N = 2 mice in each group for [1-2H]glucose infusion. For qPCR, n = 4 for control, n = 4 for ΔSHMT1, n = 5 for SHIN2 treatment, p-values from unpaired Mann-Whitney test. All mice are males.
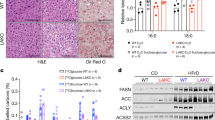
Extended Data Fig. 10 SHMT inhibition reduced sucrose-water induced hepatic lipogenesis increase.
(a) Saponified C16:0 and C18:1 concentration of serum, liver, and BAT in control and 4 weeks 20% sucrose water drinking mice (control vs. sucrose water drinking, serum p = 0.024 for C16:0, p = 0.024 for C18:1; liver p = 0.024 for C16:0, p = 0.024 for C18:1; BAT p = 0.90 for C16:0, p = 0.99 for C18:1.). (b) C16:0 and C18:1 labeling fraction in liver, brown adipose tissue, and serum from fed state 12 h D2O infusion of control and 4 weeks 20% sucrose water drinking mice. (c) Saponified C16:0 and C18:1 labeling after 4 weeks 20% sucrose water drinking in liver, brown adipose tissue, and serum with or without SHIN2 treatment after 4 weeks 20% sucrose water drinking. (d) Overall serum and liver levels of fatty acids with or without SHIN2 treatment (control vs. SHIN2 treatment, p = 0.0022 for C16:0; p = 0.0043 for C18:1). As is (g), for liver (control vs. SHIN2 treatment, p = 0.23 for C16:0, p = 0.44 for C18:1). Mean± s.d. For experiments comparing control to sucrose water drinking, n = 6 for control, n = 3 for 4-week sucrose water drinking. For experiments comparing vehicle to SHIN2 treatment, n = 6 for control and n = 7 for SHIN2. *p < 0.05; **p < 0.01; ***p < 0.005 by unpaired Mann-Whitney test. All mice are males.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Table 1.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Z., TeSlaa, T., Xu, X. et al. Serine catabolism generates liver NADPH and supports hepatic lipogenesis. Nat Metab 3, 1608–1620 (2021). https://doi.org/10.1038/s42255-021-00487-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-021-00487-4
This article is cited by
-
Leukemia inhibitory factor suppresses hepatic de novo lipogenesis and induces cachexia in mice
Nature Communications (2024)
-
Serine metabolism in macrophage polarization
Inflammation Research (2024)
-
Acetyl-CoA metabolism in cancer
Nature Reviews Cancer (2023)
-
Metabolic pathway analysis using stable isotopes in patients with cancer
Nature Reviews Cancer (2023)
-
Physiological and pathological roles of lipogenesis
Nature Metabolism (2023)