Abstract
The multifunctional roles of metabolic enzymes allow for the integration of multiple signals to precisely transduce external stimuli into cell fate decisions. Elevation of 3-phosphoglycerate dehydrogenase (PHGDH), the rate-limiting enzyme for de novo serine biosynthesis, is broadly associated with human cancer development; although how PHGDH activity is regulated and its implication in tumorigenesis remains unclear. Here we show that glucose restriction induces the phosphorylation of PHGDH by p38 at Ser371, which promotes the translocation of PHGDH from the cytosol into the nucleus. Concurrently, AMPK phosphorylates PHGDH-Ser55, selectively increasing PHGDH oxidation of malate into oxaloacetate, thus generating NADH. In the nucleus, the altered PHGDH activity restricts NAD+ level and compartmentally repressed NAD+-dependent PARP1 activity for poly(ADP-ribosyl)ation of c-Jun, thereby leading to impaired c-Jun transcriptional activity linked to cell growth inhibition. Physiologically, nuclear PHGDH sustains tumour growth under nutrient stress, and the levels of PHGDH-Ser371 and PHGDH-Ser55 phosphorylation correlate with p38 and AMPK activity, respectively, in clinical human pancreatic cancer specimens. These findings illustrate a previously unidentified nutrient-sensing mechanism with the critical involvement of a non-canonical metabolic effect of PHGDH and underscore the functional importance of alternative PHGDH activity in tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
RNA-sequencing datasets are available under accession number GSE180640. All other data are available from the authors upon reasonable request. Source data are provided with this paper.
References
Pavlova, N. N. & Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016).
DeBerardinis, R. J. & Chandel, N. S. Fundamentals of cancer metabolism. Sci. Adv. 2, e1600200 (2016).
Li, X., Egervari, G., Wang, Y., Berger, S. L. & Lu, Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat. Rev. Mol. Cell Biol. 19, 563–578 (2018).
Boon, R., Silveira, G. G. & Mostoslavsky, R. Nuclear metabolism and the regulation of the epigenome. Nat. Metab. 2, 1190–1203 (2020).
Campbell, S. L. & Wellen, K. E. Metabolic signaling to the nucleus in cancer. Mol. Cell 71, 398–408 (2018).
Fell, D. A. & Snell, K. Control analysis of mammalian serine biosynthesis. Feedback inhibition on the final step. Biochem. J. 256, 97–101 (1988).
Possemato, R. et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476, 346–350 (2011).
Locasale, J. W. et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat. Genet. 43, 869–874 (2011).
Song, Z., Feng, C., Lu, Y., Lin, Y. & Dong, C. PHGDH is an independent prognosis marker and contributes cell proliferation, migration and invasion in human pancreatic cancer. Gene 642, 43–50 (2018).
Reid, M. A. et al. Serine synthesis through PHGDH coordinates nucleotide levels by maintaining central carbon metabolism. Nat. Commun. 9, 5442 (2018).
Fan, J. et al. Human phosphoglycerate dehydrogenase produces the oncometabolite d-2-hydroxyglutarate. ACS Chem. Biol. 10, 510–516 (2015).
Kosugi, S., Hasebe, M., Tomita, M. & Yanagawa, H. Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc. Natl Acad. Sci. USA 106, 10171–10176 (2009).
Zou, Y. et al. Illuminating NAD+ metabolism in live cells and in vivo using a genetically encoded fluorescent sensor. Dev. Cell 53, 240–252 (2020).
Zhao, Y. et al. SoNar, a highly responsive NAD+/NADH sensor, allows high-throughput metabolic screening of anti-tumor agents. Cell Metab. 21, 777–789 (2015).
Zhao, Y. et al. In vivo monitoring of cellular energy metabolism using SoNar, a highly responsive sensor for NAD+/NADH redox state. Nat. Protoc. 11, 1345–1359 (2016).
Canto, C., Menzies, K. J. & Auwerx, J. NAD+ metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 22, 31–53 (2015).
Ryu, K. W. et al. Metabolic regulation of transcription through compartmentalized NAD+ biosynthesis. Science https://doi.org/10.1126/science.aan5780 (2018).
Unterlass, J. E. et al. Structural insights into the enzymatic activity and potential substrate promiscuity of human 3-phosphoglycerate dehydrogenase (PHGDH). Oncotarget 8, 104478–104491 (2017).
Weinstabl, H. et al. Intracellular trapping of the selective phosphoglycerate dehydrogenase (PHGDH) inhibitor BI-4924 disrupts serine biosynthesis. J. Med. Chem. 62, 7976–7997 (2019).
Bossy-Wetzel, E., Bakiri, L. & Yaniv, M. Induction of apoptosis by the transcription factor c-Jun. EMBO J. 16, 1695–1709 (1997).
Weiss, C. et al. JNK phosphorylation relieves HDAC3-dependent suppression of the transcriptional activity of c-Jun. EMBO J. 22, 3686–3695 (2003).
Huang, D., Wang, Y., Yang, C., Liao, Y. & Huang, K. Angiotensin II promotes poly(ADP-ribosyl)ation of c-Jun/c-Fos in cardiac fibroblasts. J. Mol. Cell. Cardiol. 46, 25–32 (2009).
Lynn, R. C. et al. c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature 576, 293–300 (2019).
Pacold, M. E. et al. A PHGDH inhibitor reveals coordination of serine synthesis and one-carbon unit fate. Nat. Chem. Biol. 12, 452–458 (2016).
Zhang, Y., Wang, J., Ding, M. & Yu, Y. Site-specific characterization of the Asp- and Glu-ADP-ribosylated proteome. Nat. Methods 10, 981–984 (2013).
Shaulian, E. & Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 4, E131–E136 (2002).
Wang, T. et al. O-GlcNAcylation of fumarase maintains tumour growth under glucose deficiency. Nat. Cell Biol. 19, 833–843 (2017).
Tao, R. et al. Genetically encoded fluorescent sensors reveal dynamic regulation of NADPH metabolism. Nat. Methods 14, 720–728 (2017).
Hall, M. D., Simeonov, A. & Davis, M. I. Avoiding fluorescence assay interference–the case for Diaphorase. Assay. Drug Dev. Technol. 14, 175–179 (2016).
Sdelci, S. et al. MTHFD1 interaction with BRD4 links folate metabolism to transcriptional regulation. Nat. Genet. 51, 990–998 (2019).
Acknowledgements
We thank L. -J. Liao at East China Normal University for the technical assistance. This work was supported by National Key R&D Program of China (2020YFA0803602 and 2017YFA0506200 to Y.J.; 2019YFA0904800 to Y.Z.), National Nature Science Foundation of China (81972586 and 81773006 to Y.J.; 32030065, 31722033 and 92049304 to Y.Z.), Shanghai Municipal Education Commission (Gaofeng Clinical Medicine grant 20161319 to Y.J.; Frontier Science Research Base of Optogenetic Techniques for Cell Metabolism grant 2021 Sci & Tech 03-28 to Y.Z.), Research Unit of New Techniques for Live-cell Metabolic Imaging (Chinese Academy of Medical Sciences, 2019-I2M-5–013 to Y.Z.), Innovative research team of high-level local universities in Shanghai, the State Key Laboratory of Bioreactor Engineering and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Contributions
This study was conceived by Y.J.; Y.J., Y.Z., C.M. and K.Z. designed the study; C.M., K.Z., K.J., Q.Z., N.S. and T.C. performed experiments; W.W. and M.Y. provided support for reviewing the manuscript; Y.J. wrote the paper with comments from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Metabolism thanks Sarah-Maria Fendt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: George Caputa.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
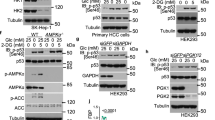
Extended Data Fig. 1 PHGDH undergoes nuclear translocation upon glucose deficiency.
In a, b, c, e, f, h-j, immunoblotting analyses were performed using the indicated antibodies. (a) SW1990 cells were transfected with control sg-RNA or 3 independent PHGDH sg-RNAs and the efficiency for PHGDH depletion was examined (upper panel); #3-PHGDH sg-RNA was selected to be utilized in the subsequent experiments; SW1990 cells with depletion of PHGDH were reconstituted with or without expression of Flag-tagged sgRNA resistant PHGDH (rPHGDH) (bottom panel). (b) Whole cellular extracts were collected from indicated cancer cell lines. (c) PANC-1 (left panel) or HUH7 (right panel) cells stably transfected with or without sg-PHGDH were cultured for 36 h in the normal or glucose-free DMEM that is supplemented with or without extra exogenous serine as indicated. Cellular viability was examined by CCK-8 assay. Data are presented as mean ± s.d. (n = 3 independent experiments), statistical analysis was performed using the two-tailed Student’s t-test. (d) SW1990 cells were cultured in the normal or glucose-free DMEM for 24 h. Levels of indicated metabolites were measured by LC-MS/MS. Data are presented as mean ± s.d. (n = 5 independent experiments). (e) LM3 cells were cultured in the glucose-free DMEM for indicated length of time. Cytosolic and nuclear extracts were collected. (f) LM3 cells expressing indicated Flag-tagged PHGDH were cultured in the glucose-free DMEM for 6 h. Nuclear extracts were collected. (g) SW1990 cells expressing indicated Flag-tagged PHGDH were cultured in the normal or glucose-free DMEM for 6 h. Nuclear extracts subjected to immunoprecipitation with an anti-Flag antibody were analyzed by coomassie brilliant blue staining (left panel). The table shows mass spectrometry-identified proteins that were specifically associated with PHGDH under glucose deprivation (right panel). (h) LM3 cells were cultured in the normal or glucose-free DMEM for 6 h. Whole cell extracts were subjected to immunoprecipitation using the indicated antibodies. (i) SW1990 cells were transfected with control shRNA or 2 independent KPNB1 shRNAs and the efficiency for KPNB1 knockdown was examined; #1-KPNB1 shRNA was selected to be utilized in the relevant experiments; (j) LM3 cells transfected with KPNB1 shRNA were cultured in the glucose-free DMEM for 6 h. Whole cellular or nuclear extracts were collected.
Extended Data Fig. 2 PHGDH Ser371 phosphorylation facilitates PHGDH nuclear translocation.
In a, b, d-g and j-m, immunoblotting analyses were performed using the indicated antibodies. (a) LM3 cells were pretreated with or without SB203580 (10 μM), Compound C (10 μM), and SP600125 (20 μM) for 1 h before being cultured in the normal or glucose-free DMEM for 6 h. Nuclear or cytosolic extracts were collected. (b) LM3 cells were pretreated with or without SB203580 (10 μM) for 1 h before being cultured in the glucose-free DMEM for 6 h. Whole cellular extracts were subjected to immunoprecipitation. (c) SW1990 cells expressing Flag-PHGDH were cultured for 6 h in the glucose-free DMEM. Immunoprecipitation analysis of nuclear extracts was performed using the Flag antibody, and the extracts were analyzed by mass spectrometry. The results of a mass spectrometric analysis of a tryptic fragment at m/z 853.44827 (mass error, +4.52 ppm) (upper panel) and 799.39655 (mass error, +1.53 ppm) (bottom panel) matched those of the doubly charged peptide, suggesting that PHGDH Ser371 and Ser55 were phosphorylated. The Sequest score for the match was Xcorr =3.85 (upper panel) and 3.61 (bottom panel). (d) SW1990 (left panel) or LM3 (right panel) cells were cultured in glucose-free DMEM with supplementation of indicated concentration of glucose for 6 h. Whole cellular extracts were collected. (e) SW1990 (left panel) or LM3 (right panel) cells were cultured in the normal or glucose-free DMEM for indicated length of time. Whole cellular extracts or nuclear extracts were collected. (f) SW1990 (upper panel) or LM3 (bottom panel) cells with depletion of PHGDH were reconstituted with or without expression of indicated Flag-tagged rPHGDH. (g) LM3 cells with depletion of PHGDH and reconstituted expression of indicated Flag–rPHGDH were cultured in the glucose-free DMEM for 6 h. Whole cellular extracts were subjected to immunoprecipitation. (h) SW1990 cells with depletion of PHGDH were reconstituted with Flag-tagged WT rPHGDH or rPHGDH S371D. Immunofluorescent analysis was performed and Flag antibody was used to detect ectopic rPHGDH; two representative fields of view were shown. (i) WT and mutant His-PHGDH were purified and the enzymatic activity for 3-PG oxidation was measured. The values are presented as mean ± s.d. (n = 3 independent experiments), statistical analysis was performed using the two-tailed Student’s t-test. (j) SW1990 (upper panel) or LM3 cells (bottom panel) were cultured in the normal or glucose-free DMEM for 6 h. Whole cellular extracts were subjected to immunoprecipitation. (k) SW1990 (upper panel) or LM3 cells (bottom panel) were pretreated with or without SB203580 (10 μM) for 1 h before being cultured in the normal or glucose-free DMEM for 6 h. (l) SW1990 cells with depletion of PHGDH and reconstituted with expression of indicated Flag–rPHGDH were cultured under normoxia or hypoxia for 12 h. Nuclear or cytosolic extracts were collected. (m) SW1990 cells were pretreated with or without SB203580 (10 μM) for 1 h before being cultured under normoxia or hypoxia for 12 h. Nuclear or cytosolic extracts were collected. (n) LM3 cells expressing with depletion of PHGDH and reconstituted with expression of indicated Flag–rPHGDH were cultured in the normal or glucose-free DMEM for 36 h. Cellular viability was examined by CCK-8 assay. The values are presented as mean ± s.d. (n = 3 independent experiments), statistical analysis was performed using the two-tailed Student’s t-test. (o) The cartoon showing PHGDH is phosphorylated at Ser371 by p38 upon glucose deficiency; this phosphorylation promotes PHGDH nuclear translocation. At this condition, PHGDH phosphorylation at Ser55 is also detected, while the relevant physiological role remains unclear at this stage.
Extended Data Fig. 3 PHGDH negatively regulates nuclear NAD+ upon glucose deprivation.
In g-i, immunoblotting analyses were performed using the indicated antibodies. In a-f, data are presented as mean ± s.d. (n = 3 independent experiments), statistical analysis was performed using the two-tailed Student’s t-test. (a) SW1990 cells with or without depletion of PHGDH were transfected with nucleus-targeting sequence containing mCherry-FiNad (Nuc-mCherry-FiNad) and mCherry-cpYFP (Nuc-mCherry-cpYFP) (left panel) or SoNar (Nuc-SoNar) and iNapc (Nuc-iNapc) (right panel). Cells were cultured in the normal or glucose-free DMEM for 12 h. Normalized ratio of fluorescence intensities excited at 485 nm and 590 nm (F485nm/F590nm indicates NAD+) and fluorescence intensities excited at 420 nm and 485 nm (F420nm/F485nm indicates NADH/NAD+) were recorded by Microplate Reader. (b) SW1990 (left panel) or LM3 (right panel) cells with depletion of PHGDH and reconstituted with indicated Flag-tagged rPHGDH were cultured in the normal or glucose-free DMEM for 36 h. Cellular viability was examined by CCK-8 assay. (c, d) SW1990 cells with or without depletion of PHGDH (c) or SW1990 cells with depletion of PHGDH and reconstituted with indicated Flag-tagged rPHGDH (d) were cultured in the normal or glucose-free DMEM for 12 h. The NAD+ (left panels) and NADH/NAD+ (right panels) content was measured using the NAD+ assay kit. (e, f) SW1990 cells with or without PHGDH depletion (e) or with depletion of PHGDH and reconstituted expression of indicated Flag–rPHGDH (f) were transfected with mCherry-FiNad and mCherry-cpYFP (left panels) or SoNar and iNapc (right panels). Cells were cultured in the normal or glucose-free DMEM for 12 h. Normalized ratio of fluorescence intensities was recorded by Microplate Reader. (g) SW1990 (left panel) or LM3 (right panel) cells with or without depletion of PHGDH were cultured in the normal or glucose-free DMEM for 12 h. (h) LM3 cells with depletion of PHGDH and reconstituted with indicated Flag-tagged rPHGDH were cultured in the normal or glucose-free DMEM for 12 h. (i) SW1990 cells with or without depletion of PHGDH were transfected with or without si-NMANT1. Cells were cultured in the glucose-free DMEM for 12 h.
Extended Data Fig. 4 AMPK phosphorylates PHGDH and promotes PHGDH-catalyzed malate oxidation.
In b, c, d, f and h, immunoblotting analyses were performed using the indicated antibodies. In a, b, g, i-k, data are presented as mean ± s.d. (n = 3 independent experiments), statistical analysis was performed using the two-tailed Student’s t-test. (a, b) SW1990 cells with depletion of PHGDH and reconstituted with indicated Flag-tagged rPHGDH were cultured in the normal or glucose-free DMEM for 12 h. The enzymatic activity for 3-PG and 2-HG (a) or 3-PG, malate and 2-HG (b) oxidation of immunoprecipitated-rPHGDH proteins was determined. (c) SW1990 cells were cultured in the normal or glucose-free DMEM for 12 h. Ser55-phosphorylated (left panel) or Ser371-phosphorylated (right panel) PHGDH was depleted using the indicated antibodies. Whole cell lysates were collected. (d) SW1990 (left panel) or LM3 (right panel) cells were cultured in the normal or glucose-free DMEM for 12 h. Ser55-phosphorylated PHGDH was depleted using the indicated antibodies. Nuclear extracts were collected. (e) The cartoon showing the status of PHGDH nuclear accumulation and activity under glucose deficiency. When p38 and AMPK are both activated under glucose deficiency, PHGDH Ser371 and Ser55 can be independently phosphorylated at PHGDH, or concomitantly phosphorylated at PHGDH, among which PHGDH with both phosphorylation account for a larger part. PHGDH Ser55 phosphorylation by AMPK promotes the catalytic activity of PHGDH for malate oxidation. In this way, PHGDH with Ser371 and Ser55 phosphorylation can translocate into the nucleus and exhibits the enhanced activity for malate oxidation, which thereby represses nuclear NAD+. (f-i) In vitro kinase assays were performed by incubating bacterial purified recombinant His-WT PHGDH or His-PHGDH S55A (f and g) and His-WT PHGDH or PHGDH S371D (h and i) with or without purified AMPK complex. Immunoblotting analysis was performed (f and h). The enzymatic activity of pulldown PHGDH for malate (g and i, left panels) or 3-PG (g and i, right panels) oxidation was determined. (j, k) SW1990 cells expressing indicated Flag-rPHGDH was orderly supplemented with 0, 0.5 mM, 2.5 mM and 5 mM diethyl-malate. Cells were cultured for 12 h in the glucose-free DMEM. The relative level of malate (j), NAD+ (k, left panel) or NADH/ NAD+ (k, right panel) was measured.
Extended Data Fig. 5 Nuclear PHGDH represses c-Jun PARylation and transcriptional activity.
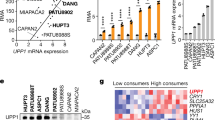
In e-j and m-o, immunoblotting analyses were performed using the indicated antibodies. In b, d and k, mRNA levels of potential c-Jun targeted genes were analyzed by real–time PCR. In b, d, k, l, p and q, data are presented as mean ± s.d. (n = 3 independent experiments), statistical analysis was performed using the two-tailed Student’s t-test. (a) Gene ontology (GO) analysis of the changed genes in the WT rPHGDH group versus rPHGDH S371A group as shown in Fig. 5a. (b) SW1990 cells with depletion of PHGDH and reconstituted with indicated Flag-tagged rPHGDH were transfected with c-Jun siRNA. Cells were cultured in the glucose-free DMEM for 24 h. (c) The cDNA microarray analysis was performed for WT rPHGDH and rPHGDH S55A-expressing cells cultured in the glucose-free DMEM for 24 h. Among genes that are differentially expressed between two groups, the genes with similar tendency to that were displayed in Fig. 5a were shown. (d) SW1990 cells with depletion of PHGDH and reconstituted with indicated Flag-tagged rPHGDH were transfected with c-Jun siRNA. Cells were cultured in the glucose-free DMEM for 24 h. (e) LM3 cells with depletion of PHGDH and reconstituted with indicated rPHGDH were cultured in the normal or glucose-free DMEM for 12 h (left panel). LM3 cells expressing indicated Flag-rPHGDH were pretreated with or without PJ34 (20 μM) and were cultured in the glucose-free DMEM for 12 h (right panel). Whole cellular extracts were subjected to immunoprecipitation using the indicated antibodies. (f, g) SW1990 (left panels) or LM3 (right panels) cells with depletion of PHGDH and reconstituted with indicated rPHGDH were transfected with or without si-NMNAT1 (f) or pretreated with or without FK866 (10 nM) for 1 h (g). Whole cellular extracts were subjected to immunoprecipitation using the indicated antibodies. (h) SW1990 cells were transfected with control sg-RNA or two independent c-Jun sgRNAs and the efficiency of c-Jun depletion was examined (top); #2-c-Jun sgRNA was selected to be used in the subsequent experiments. The simplified schematic showing c-JunΔD with deletion of the c-Jun DNA-binding region (middle). SW1990 cells with depletion of c-Jun were reconstituted with expression of indicated Flag–rc-Jun (bottom). (i) SW1990 cells with depletion of c-Jun were reconstituted with expression of indicated sgRNA resistant Flag-c-Jun (Flag-rc-Jun). Soluble or chromatin-associated fraction was collected. (j) SW1990 cells expressing indicated Flag-rc-Jun were pretreated with NCT503 (40 μM) and were cultured in the glucose-free DMEM for 12 h. Whole cellular extracts were subjected to immunoprecipitation using the indicated antibodies. (k) SW1990 cells expressing indicated Flag-rc-Jun proteins were pretreated with NCT503 (40 μM). Cells were cultured in the normal or glucose-free DMEM for 24 h. (l) SW1990 cells expressing Flag-PHGDH were pretreated with NCT503 (40μM). Cells were cultured in the normal or glucose-free DMEM for 12 h. The enzymatic activity for 3-PG (left panel) or Malate (right panel) oxidation of immunoprecipitated-PHGDH proteins was determined. (m) SW1990 cells expressing indicated Flag-c-Jun were pretreated with or without NCT-503 (40 μM). Cells were cultured in the glucose-free DMEM for 12 h. Whole cellular extracts were subjected to immunoprecipitation using the indicated antibodies. (n) SW1990 (upper panel) or LM3 (bottom panel) cells with depletion of c-Jun were reconstituted with expression of indicated Flag–rc-Jun. (o) SW1990 cells expressing indicated Flag-c-Jun were pretreated with NCT-503 (40 μM) and were cultured in the glucose-free DMEM for 24 h. Chromatin-associated fraction was collected. (p) SW1990 cells expressing indicated Flag-rPHGDH were cultured in the normal or glucose-free DMEM for 24 h. Luciferase reporter assays were performed. (q) Simplified schematic showing the putative c-Jun binding site in the GADD45B or RARRES3 promoter; the consensus and mutant sequences for c-Jun binding are red marked (upper panel). SW1990 cells with depletion of PHGDH and indicated Flag-rPHGDH were cultured in the glucose-free DMEM for 24 h. Luciferase reporter assays were performed (bottom panel).
Extended Data Fig. 6 PHGDH interacts with S73 phosphorylated-c-Jun and represses c-Jun PARylation.
In b-f, h, l and m, whole cellular extracts were subjected to immunoblotting analyses using the indicated antibodies. In g-k and n-p, data are presented as mean ± s.d. (n = 3 independent experiments), statistical analysis was performed using the two-tailed Student’s t-test. (a) SW1990 cells expressing Flag-PHGDH were cultured for 6 h in the glucose-free DMEM. Immunoprecipitation analysis of nuclear extracts was performed using the Flag antibody, and the extracts were analyzed by mass spectrometry. The results of a mass spectrometric analysis of a tryptic fragment at m/z 497.73694 (mass error, +6.08 ppm) matched those of the doubly charged peptide, suggesting that c-Jun Ser73 was phosphorylated. The Sequest score for the match was Xcorr =0.79. (b) SW1990 (upper panel) or LM3 cells (bottom panel) with depletion of c-Jun were reconstituted with expression of indicated Flag-tagged rc-Jun. (c) LM3 cells with depletion of c-Jun and reconstituted with indicated Flag-rc-Jun were cultured in the normal or glucose-free DMEM for 6 h. Whole cellular extracts were subjected to immunoprecipitation using the indicated antibodies. (d) The in vitro kinase assay was performed with incubation of indicated purified c-Jun with or without of immunoprecipitated JNK1; then c-Jun was collected and incubated with PARP1/DNA/NAD+ mixture to conduct in vitro PARylation assay (upper panel). The in vitro PARylation assay was first performed with incubation of indicated c-Jun with or without PARP1/DNA/NAD+ mixture and then c-Jun was collected, which was followed by in vitro kinase assay with immunoprecipitated JNK1 (bottom panel). (e) SW1990 cells with depletion of PHGDH and reconstituted with expression of indicated Flag-rPHGDH were cultured in the normal or glucose-free DMEM for 6 h. Whole cellular extracts were subjected to immunoprecipitation using the indicated antibodies. (f) SW1990 cells with depletion of PHGDH and reconstituted with expression of indicated Flag-rPHGDH were cultured in the normal or glucose-free DMEM for 12 h (upper panel). SW1990 cells expressing indicated Flag-rPHGDH were cultured in the glucose-free DMEM for 12 h, S73-phosphorylated c-Jun was depleted using the indicated antibodies (bottom panel). Chromatin extracts were collected. (g) SW1990 (left panel) or LM3 (right panel) cells transfected with or without c-Jun sgRNA were pretreated with or without NCT-503 (40 μM). Cells were cultured in the normal or glucose-free DMEM for 36 h. Cellular viability was examined by CCK-8 assay. (h) SW1990 (left panel) or LM3 (right panel) cells expressing indicated Flag-rPHGDH were transfected with or without c-Jun siRNA. Cells were cultured in the normal or glucose-free DMEM for 36 h. Cellular viability was examined by CCK-8 assay. (i) LM3 cells with depletion of c-Jun and reconstituted with indicated Flag-rc-Jun were pretreated with or without NCT-503 (40 μM). Cells were cultured in the normal or glucose-free DMEM for 36 h. Cellular viability was examined by CCK-8 assay. (j, k) SW1990 (j) or LM3 (k) cells with depletion of PHGDH and reconstituted with expression of indicated Flag-rPHGDH were pretreated with or without SP600125 (20 μM). Cells were cultured in the normal or glucose-free DMEM for 36 h. (l, m) SW1990 (l) or LM3 (m) cells pretreated with or without SP600125 (20 μM) were cultured in the normal or glucose-free DMEM for 12 h. (n) LM3 cells with depletion of c-Jun and reconstituted with expression of indicated Flag–rc-Jun were cultured in the normal or glucose-free DMEM for 36 h. Cellular viability was examined by CCK-8 assay. (o, p) SW1990 (o) or LM3 (p) cells with depletion of PHGDH were pretreated with or without PJ-34 (20 μM). Cells were cultured in the normal or glucose-free DMEM for 36 h. Cellular viability was examined by CCK-8 assay.
Extended Data Fig. 7 Nuclear PHGDH promotes tumorigenesis.
In a-h, a total of 1 × 107 SW1990 (a and c) or 5 × 106 LM3 (b, d-h) cells expressing PHGDH sg-RNA (a and b) or c-Jun sg-RNA (h) and indicated Flag-rPHGDH (c-e) or Flag-rc-Jun (f-g) were subcutaneously injected into the athymic nude mice. Tumor volumes were measured by using length (a) and width (b) and calculated using the following equation: V = ab2/2. Data represent the mean ± s.d. (n = 7), statistical analysis was performed using the two-tailed Student’s t-test. (a-e) Representative tumor xenografts (left panels) and quantification of tumor volumes were shown (middle panels); lysates harvested from SW1990 or LM3 cells that were cultured for 6 h in the glucose-free DMEM and tumor tissues were subjected to immunoblotting analyses (right panels). (f-h) NCT503 (40 mg/kg/day) was used to administrate mice 7 days post subcutaneous injection of tumor cells. Representative tumor xenografts (left panels) and quantification of tumor volumes were shown (middle panels); lysates were harvested from LM3 cells that were cultured for 6 h in the glucose-free DMEM and tumor tissues were subjected to immunoprecipitation or immunoblotting analyses (right panels). (i) The PHGDH pS371 (upper panel) and PHGDH pS55 (bottom panel) antibodies specificities were validated using IHC analyses with specific blocking PHGDH pS371 or PHGDH pS55 peptides. Scale bar: 50 μm.
Supplementary information
Source data
Source Data Fig. 1
Unprocessed western blots and/or source data.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Unprocessed western blots and/or gels.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Unprocessed western blots and/or gels.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Unprocessed western blots and/or gels.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Unprocessed western blots and/or gels.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Unprocessed western blots and/or gels.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Unprocessed western blots and/or gels.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
About this article
Cite this article
Ma, C., Zheng, K., Jiang, K. et al. The alternative activity of nuclear PHGDH contributes to tumour growth under nutrient stress. Nat Metab 3, 1357–1371 (2021). https://doi.org/10.1038/s42255-021-00456-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-021-00456-x
This article is cited by
-
Cellular metabolism regulates the differentiation and function of T-cell subsets
Cellular & Molecular Immunology (2024)
-
Metabolic heterogeneity in cancer
Nature Metabolism (2024)
-
Comprehensive analysis of PHGDH for predicting prognosis and immunotherapy response in patients with endometrial carcinoma
BMC Medical Genomics (2023)
-
An idle PHGDH takes control of cell fate
Cell Research (2023)
-
IGF1R-phosphorylated PYCR1 facilitates ELK4 transcriptional activity and sustains tumor growth under hypoxia
Nature Communications (2023)