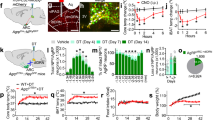
Abstract
Hypothalamic agouti-related peptide (AgRP) and neuropeptide Y-expressing neurons have a critical role in driving food intake, but also in modulating complex, non-feeding behaviours1. We interrogated whether AgRP neurons are relevant to the emergence of anorexia nervosa symptomatology in a mouse model. Here we show, using in vivo fibre photometry, a rapid inhibition of AgRP neuronal activity following voluntary cessation of running. All AgRP neuron-ablated, food-restricted mice die within 72 h of compulsive running, while daily activation of AgRP neurons using a chemogenetic tool increases voluntary running with no lethality of food-restricted animals. Animals with impaired AgRP neuronal circuits are unable to properly mobilize fuels during food-restriction-associated exercise; however, when provided with elevated fat content through diet, their death is completely prevented. Elevated fat content in the diet also prevents the long-term behavioural impact of food-restricted fit mice with elevated exercise volume. These observations elucidate a previously unsuspected organizational role of AgRP neurons, via the mediation of the periphery, in the regulation of compulsive exercise and its related lethality with possible implications for psychiatric conditions, such as anorexia nervosa.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Change history
25 January 2021
A Correction to this paper has been published: https://doi.org/10.1038/s42255-021-00351-5.
References
Dietrich, M. O., Zimmer, M. R., Bober, J. & Horvath, T. L. Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell 160, 1222–1232 (2015).
Luquet, S., Perez, F. A., Hnasko, T. S. & Palmiter, R. D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685 (2005).
Aponte, Y., Atasoy, D. & Sternson, S. M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 14, 351–355 (2011).
Betley, J. N., Cao, Z. F., Ritola, K. D. & Sternson, S. M. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155, 1337–1350 (2013).
Krashes, M. J. et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 121, 1424–1428 (2011).
Dietrich, M. O. et al. AgRP neurons regulate development of dopamine neuronal plasticity and nonfood-associated behaviors. Nat. Neurosci. 15, 1108–1110 (2012).
Li, C. et al. AGRP neurons modulate fasting-induced anxiolytic effects. Transl. Psychiatry 9, 111 (2019).
Sestan-Pesa, M. & Horvath, T. L. Metabolism and mental illness. Trends Mol. Med. 22, 174–183 (2016).
Treasure, J. et al. Anorexia nervosa. Nat. Rev. Dis. Primers. 1, 15074 (2015).
Levallius, J., Collin, C. & Birgegard, A. Now you see it, now you don’t: compulsive exercise in adolescents with an eating disorder. J. Eat. Disord. 5, 9 (2017).
Lichtenstein, M. B., Hinze, C. J., Emborg, B., Thomsen, F. & Hemmingsen, S. D. Compulsive exercise: links, risks and challenges faced. Psychol. Res. Behav. Manag. 10, 85–95 (2017).
Routtenberg, A. & Kuznesof, A. W. Self-starvation of rats living in activity wheels on a restricted feeding schedule. J. Comp. Physiol. Psychol. 64, 414–421 (1967).
Klenotich, S. J. & Dulawa, S. C. The activity-based anorexia mouse model. Methods Mol. Biol. 829, 377–393 (2012).
Hahn, T. M., Breininger, J. F., Baskin, D. G. & Schwartz, M. W. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat. Neurosci. 1, 271–272 (1998).
Takahashi, K. A. & Cone, R. D. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/agouti-related protein neurons. Endocrinology 146, 1043–1047 (2005).
Dana, H. et al. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat. Methods 16, 649–657 (2019).
Zimmer, M. R., Fonseca, A. H. O., Iyilikci, O., Pra, R. D. & Dietrich, M. O. Functional ontogeny of hypothalamic Agrp neurons in neonatal mouse behaviors. Cell 178, 44–59 (2019).
He, Z. et al. Cellular and synaptic reorganization of arcuate NPY/AgRP and POMC neurons after exercise. Mol. Metab. 18, 107–119 (2018).
Chen, Y., Lin, Y. C., Kuo, T. W. & Knight, Z. A. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160, 829–841 (2015).
Tan, K., Knight, Z. A. & Friedman, J. M. Ablation of AgRP neurons impairs adaption to restricted feeding. Mol. Metab. 3, 694–704 (2014).
Cavalcanti-de-Albuquerque, J. P., Bober, J., Zimmer, M. R. & Dietrich, M. O. Regulation of substrate utilization and adiposity by Agrp neurons. Nat. Commun. 10, 311 (2019).
Cahill, G. F. Jr. Fuel metabolism in starvation. Annu Rev. Nutr. 26, 1–22 (2006).
Wang, Q. et al. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol. Metab. 17, 64–72 (2013).
Chen, Y. W., Surgent, O., Rana, B. S., Lee, F. & Aoki, C. Variant BDNF-Val66Met polymorphism is associated with layer-specific alterations in GABAergic innervation of pyramidal neurons, elevated anxiety and reduced vulnerability of adolescent male mice to activity-based anorexia. Cereb. Cortex 27, 3980–3993 (2017).
Wable, G. S., Min, J. Y., Chen, Y. W. & Aoki, C. Anxiety is correlated with running in adolescent female mice undergoing activity-based anorexia. Behav. Neurosci. 129, 170–182 (2015).
Rodgers, R. J. & Dalvi, A. Anxiety, defence and the elevated plus-maze. Neurosci. Biobehav. Rev. 21, 801–810 (1997).
Deacon, R. M. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat. Protoc. 1, 122–124 (2006).
Gyertyan, I. Analysis of the marble burying response: marbles serve to measure digging rather than evoke burying. Behav. Pharmacol. 6, 24–31 (1995).
Witkin, J. M. Animal models of obsessive–compulsive disorder. Curr. Protoc. Neurosci. Chapter 9, Unit 9.30 (2008).
Joly-Amado, A. et al. Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. EMBO J. 31, 4276–4288 (2012).
Padilla, S. L. et al. Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat. Neurosci. 19, 734–741 (2016).
Halmi, K. A. et al. Obsessions and compulsions in anorexia nervosa subtypes. Int. J. Eat. Disord. 33, 308–319 (2003).
Thiel, A., Broocks, A., Ohlmeier, M., Jacoby, G. E. & Schussler, G. Obsessive-compulsive disorder among patients with anorexia nervosa and bulimia nervosa. Am. J. Psychiatry 152, 72–75 (1995).
Moriya, J., Takimoto, Y., Yoshiuchi, K., Shimosawa, T. & Akabayashi, A. Plasma agouti-related protein levels in women with anorexia nervosa. Psychoneuroendocrinology 31, 1057–1061 (2006).
Sarrar, L. et al. Cognitive flexibility and agouti-related protein in adolescent patients with anorexia nervosa. Psychoneuroendocrinology 36, 1396–1406 (2011).
Siegfried, Z., Berry, E. M., Hao, S. & Avraham, Y. Animal models in the investigation of anorexia. Physiol. Behav. 79, 39–45 (2003).
Watson, H. J. et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat. Genet. 51, 1207–1214 (2019).
Duncan, L. et al. Significant locus and metabolic genetic correlations revealed in genome-wide association study of anorexia nervosa. Am. J. Psychiatry 174, 850–858 (2017).
Ruan, H. B. et al. O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat. Cell 159, 306–317 (2014).
Festing, M. F. On determining sample size in experiments involving laboratory animals. Lab Anim. 52, 341–350 (2018).
Acknowledgements
This work was partly funded by the Swiss National Science Foundation (Early Postdoc.Mobility P2BEP3_172252 to M.C.M.), NIDDK grant R01DK107916 (to M.O.D.), National Institutes of Health grants AG052005 and DK111178, and a Klarman Family Foundation grant (to T.L.H.). T.L.H. was also supported by grant NKFI-126998 from the Hungarian National Research, Development and Innovation Office. We thank the Janelia GENIE project for the jGCaMP7s plasmid. The authors thank D. Giordano (Starr Life Sciences) for the extraordinary technical support with the running wheel equipment and A. Feier for his attentive animal work.
Author information
Authors and Affiliations
Contributions
T.L.H. developed the concept; M.C.M. developed the experimental strategy with input from T.L.H.; O.I. and M.O.D. developed and performed fibre photometry experiments; M.C.M., M.S., M.S.-P. and A.C. conducted experiments; M.C.M. analysed data; C.J.Z. evaluated necropsy material; and M.C.M. and T.L.H. wrote the paper with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: Christoph Schmitt.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Scheme of the activity-based anorexia (ABA) model.
a, Scheme of the activity-based anorexia (ABA) model with timing/doses of diphtheria toxin for AgRPDTR mice or capsaicin injections for AgRPTRPV1 mice. b, During food restriction, mice have access to ad lib food from 7:00 pm to 9:00 pm.
Extended Data Fig. 2 Ablation of AgRP neurons by diphtheria toxin (DT) injections on P5 in the arcuate nucleus.
Both control and AgrpDTR+/+ mice were injected as pups with DT (50 μg/kg) on P5. Representative immunostaining of ARC neurons of control (a), Heterozygous mice AgRPDTR+/–Het (b) and Homozygous mice AgRPDTR+/+-Homo (c) are shown. *The asterisks indicate third ventricle. Scale bar, 50 μm. Wild-type AgRPDTR-/- n = 3, Heterozygous AgRPDTR+/- n=4, Homozygous AgRPDTR+/+ n=3.
Extended Data Fig. 3 Histologic findings in running-AgRPDTR animals (a, c, e, g) and sedentary-AgRPDTR mice (b, d,f, h) fed standard diet at the end of the ABA paradigm.
a, b, Interscapular fat and junction of brown (BAT) and white (WAT) adipose tissue. Both BAT (black asterisk) and WAT (white asterisk) stores are depleted in running-AgRPDTR fed standard diet but not in -sedentaryAgRPDTR mice. c, d, Mesenteric fat: Marked atrophy of mesenteric fat stores are evident in the running-AgRPDTR animals (white asterisks) but not in sedentary-AgRPDTR mice. e, f, Skin, dorsum: Marked atrophy of subcutaneous fat stores are evident in running-AgRPDTR animals but not in sedentary-AgRPDTR mice (white asterisks). g, h, Skeletal muscle, quadriceps femoris: Marked myofiber atrophy is evident in the running-AgRPDTR animals but not in sedentary-AgRPDTR mice. Hematoxylin and eosin, Bar = 100μm (a, b), 50 μm (c-h). R-AgRPDTR n=3, S-AgRPDTR n=6.
Extended Data Fig. 4 Histologic findings in running-AgRPDTR animals (a,c,e,g) and sedentary-AgRPDTR mice (b, d, f, h) fed high fat diet at the end of the ABA paradigm.
a,b, Perirenal fat: Both BAT (black asterisk) and WAT (white asterisk) stores are comparable between running and sedentary AgRPDTR mice. c,d, Mesenteric fat: Mesenteric fat stores are similar in running and sedentary AgRPDTR mice. e,f, Skin, dorsum: Comparable subcutaneous white adipose tissue is evident in running-AgRPDTR mice compared to sedentary AgRPDTR mice. (asterisk). g,h, Skeletal muscle, quadriceps femoris: Myofiber morphology is comparable between running and sedentary AgRPDTR mice Hematoxylin and eosin, Bar = 100 μm (a, b, e, f), 50 μm (c, d, g, h) R-AgRPDTR n=3, S-AgRPDTR n=4.
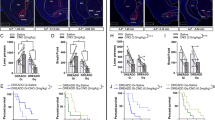
Extended Data Fig. 5 Male mice exhibit same phenotype as females under ABA.
All studies have been conducted in AgRPDTR male mice and controls (left) and AgRPTRPV1 mice and control (P36-P50) (right). Sedentary mice (called S-AgRPDTR, S-AgRpTRPV1 and S-control respectively) underwent food restriction without access to the running wheel. Only data for male mice are shown. Data are expressed as mean ± s.e.m. A: acclimation, FR: food restriction, R: recovering. R-AgRPDTR n=14, S- AgRPDTR =14, R-control n=13, S-control=11. R-AgRPTRPV1 n=11, S- AgRPTRPV1=12, R-control=14, S-control n=14, A: Body weight changes during ABA, Day 5: R-AgRPDTR versus S-control, **P=0.0047; S-control vs R-AgRPTRPV1 ***P=0.0001, Day 6: R-AgRPDTR vs S-AgRPDTR and vs S-control ***P=0.0001, R-AgRPTRPV1 vs S-control and vs S-AgRPTRPV1 ***P=0.0001, Day 7: R-AgRPDTR vs S-AgRPDTR and vs S-control; ***P=0.0001, R-AgRPTRPV1 vs S-control and vs S-AgRPTRPV1 ***P=0.0001, B: Caloric intake during acclimation (Kcal in 24 hr), No statistical differences across groups were found. C: Caloric intake during food restriction (Kcal in 2 hr), Two-way Anova followed by Tukey’s multiple comparisons test. Day 3: R-AgRPDTR vs other groups ***P=0.0001. D: Daily running wheel activity during acclimation and food restriction. Two-way Anova followed by Sidak’s multiple comparisons test. Day 6: R-control vs R-AgRPDTR, ***P=0.0001; R-control vs R-AgRPTRPV1 **P=0.0088, Day 7: R-control vs R-AgRPDTR, ***P=0.0008; R-control vs R-AgRPTRPV1 ***P=0.0001. E: Survival during food restriction. Long-rank test for comparison of survival curves: R-control vs R-AgRPDTR **P=0.0042; R-control vs R-AgRPTRPV1 :P ≥0.9999.
Extended Data Fig. 6 Minimal caloric intake pair-feeding studies.
Mice with intact or activated AgRP neuron pair-fed to the level that AgRP-DTR animals ate on standard diet ran and survived with minimal caloric intake (left). 1 Kcal of HFD provided during food restriction was able to rescue survival of mice with ablated AgRP neurons (right). All studies were conducted in AgRPTRPV1 female mice and control (n=6 per group), or AgRPDTR female mice (n=5 per group) and control (P36-50). Data are expressed as mean ± s.e.m. No statistical differences were found among groups. A: acclimation, FR: food restriction, R: recovering. a,b, Body weight changes during ABA. c,d Caloric intake during food restriction (Kcal in 2 hr). e,f Daily running wheel activity during acclimation and food restriction. g,h Survival rate during food restriction.
Extended Data Fig. 7 Ablation of AgRP neurons impairs metabolic adaptation to ABA paradigm.
All studies were conducted in control and AgRPDTR mice (left) or control and AgRPTRPV1 mice (P36-P50) (right) .Sedentary mice (named S-AgRPDTR, S-AgRPTRPV1 and S-control respectively) underwent food restriction without access to the running wheel. Only Data for female mice are shown. Data are expressed as mean ± s.e.m. R-AgRPDTR n=16, S- AgRPDTR =14, R-control n=16, S-control=12. R-AgRPTRPV1 n=11, S- AgRPTRPV1=13, R-control=12, S-control n=12. Whiskers box goes from the smallest values to the largest and extends from the 25th to 75th percentiles. a, Serum Free Fatty Acid (FFA) (μM in serum) R-AgRPDTR vs all other groups **** P< 0.0001 (24 hr, 48 hr and 72 hr food restriction). AgRPTRPV1 mice, 24 hr food restriction: R-control vs S-AgRPTRPV1 * P=0.0426. 48 hr food restriction; R-control vs R-AgRPTRPV1; * P=0.0225, S-control vs R-AgRPTRPV1. * P=0.0169; R-AgRPTRPV1 vs S-AgRPTRPV1 ****P< 0.0001. b, Serum ketone bodies (mmol/L). AgRPDTR mice on the left: 24 hr food restriction: R-control vs R-AgRPDTR ** P=0.0013, S-control vs R-AgRPDTR **** P< 0.0001; R-AgRPDTR vs. S-AgRPDTR P< 0.0001. 48 hr food restriction: R-control vs S-control * P=0.0387; R-control vs S-AgRPDTR * P=0.0175: S-control vs R-AgRPDTR *** P=0.0004; R-AgRPDTR vs S-AgRPDTR *** P=0.0001. 72 hr food restriction: R-control vs R-AgRPDTR ** P=0.0022, S-control vs S-control AgRPDTR * P=0.0307; R-AgRPDTR vs S-AgRPDTR **** P< 0.0001. AgRPTRPV1 mice (on the right) 24 hr food restriction: R-control vs S-control ** P=0.0043; R-control vs R-AgRP TRPV1 *** P=0.0004: R-control vs S-AgRP TRPV1 **** P< 0.0001. 48 hr food restriction : R-control vs R-AgRP TRPV1 **** P< 0.0001; R-control vs S-AgRP TRPV1**** P< 0.0001 ; S-control vs R-AgRP TRPV1 ** P=0.0043; S-control vs S-AgRP TRPV1**** P< 0.0001, 72 hr food restriction :R-control vs R-AgRP TRPV1 **** P< 0.0001, R-control vs S-AgRP TRPV1 **** P< 0.0001, S-control vs R-AgRP TRPV1 *** P=0.0007. c, Glucose concentrations (mg/dl). R-AgRPDTR vs R-control ** P=0.0062 (24 hr food restriction). R-AgRPDTR vs all other groups **** P< 0.0001 (48 hr and 72 hr food restriction). R-AgRPTRPV1 vs S-AgRP TRPV1: * P=0.0382 (24 hr food restriction).
Extended Data Fig. 8 Serum concentrations of active ghrelin (pg/ml).
Active ghrelin (pg/ml) was measured in serum by Elisa, Data are expressed as mean ± s.e.m. Two-way ANOVA followed by a Tukey’s multiple comparisons test. R-AgRPDTR n=4, S- AgRPDTR =4, R-control n=4, S-control=4. R-AgRPTRPV1 n=4, S- AgRPTRPV1=4, R-control=4, S-control n=4. a, b, Ghrelin concentrations in female and male AgRPDTR- and controls. Females: in all groups 24 hr vs fed ****P< 0.0001. 48 hr vs 24 hr S-control **P=0.0021, 48 hr vs 24 hr S-AgRPDTR ***P=0.0010, R- AgRPDTR 24 hr vs 48 and 72 hr ****P< 0.0001. Males: in all groups 24 hr vs fed ****P< 0.0001. S-AgRPDTR24 hr vs 48 hr ***P=0.0009; R- AgRPDTR 24 hr vs 48 and 72 hr ****P< 0.0001. C-D) Ghrelin concentrations in female and male AgRPTRPV1- and controls . Females: in all groups 24 hr vs fed ****P< 0.0001, R-control 24 hr vs 48 and 72 hr ***P=0.0004. Male: in all groups 24 hr vs fed ****P< 0.0001, R- control 24 hr vs 48 and 72 hr ***P=0.0001. R- AgRPTRPV1 24 hr vs 48 and 72 hr ***P= 0.0001.
Extended Data Fig. 9 High fat diet rescues normal survival and prevents ABA phenotype development in male mice.
All studies have been conducted in control and AgRPDTR male mice (left) and AgRPTRPV1 mice and control mice (P36-P50) (right). Only data for male mice are shown. Data are expressed as mean ± s.e.m. SD: standard diet. HFD: high fat diet. A: acclimation, FR: food restriction, R: recovering. R-AgRPDTR SD n=14, R-AgRPDTR HFD n=13. R-AgRPTRPV1 n=12, R-AgRPTRPV1 n=13. a, Body weight changes during ABA Two-way Anova followed by Sidak’s multiple comparisons test. Day 6: R-AgRPDTR SD vs R-AgRPDTR HFD, ***P=0.0001. Day 7: R-AgRPDTR SD vs R-AgRPDTR HFD, ***P=0.0001. b, Caloric intake during acclimation (Kcal in 24 hr). Day 1, 2 and 3: R-AgRPDTR SD vs R-AgRPDTR HFD, ***P=0.0001; R-AgRPTRPV1 SD vs R-AgRPTRPV1 HFD, ***P=0.0001, c, Caloric intake during food restriction (Kcal in 2 hr). Two-way Anova followed by Tukey’s multiple comparisons test. Day 1, 2 and 3: R-AgRPDTR SD vs R-AgRPDTR HFD, ***P=0.0001; R-AgRPTRPV1 SD vs R-AgRPTRPV1 HFD, ***P=0.0001. d, Daily running wheel activity during acclimation and food restriction. Two-way Anova followed by Sidak’s multiple comparisons test.. Day 6: R-AgRPDTR SD vs R-AgRPDTR HFD ***P=0.0001, Day 7: R-AgRPDTR SD vs R-AgRPDTR HFD, ***P=0.0001. Day 9: R-AgRPTRPV1 SD vs R-AgRPTRPV1 HFD, ***P=0.0008, Day 10: R-AgRPTRPV1 SD vs R-AgRPTRPV1 HFD, ***P=0.0001 Survival during food restriction. e, Long-rank test for comparison of survival curves: R-AgRPDTR SD vs R-AgRPDTR HFD **P=0.0093 R-control vs R-AgRPTRPV1 no statistical difference, P ≥0.9999.
Supplementary information
Rights and permissions
About this article
Cite this article
Miletta, M.C., Iyilikci, O., Shanabrough, M. et al. AgRP neurons control compulsive exercise and survival in an activity-based anorexia model. Nat Metab 2, 1204–1211 (2020). https://doi.org/10.1038/s42255-020-00300-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-020-00300-8
This article is cited by
-
Effects of lifestyle factors on leukocytes in cardiovascular health and disease
Nature Reviews Cardiology (2024)
-
microRNA-33 controls hunger signaling in hypothalamic AgRP neurons
Nature Communications (2024)
-
Identification of adipose tissue transcriptomic memory of anorexia nervosa
Molecular Medicine (2023)
-
AgRP neurons coordinate the mitigation of activity-based anorexia
Molecular Psychiatry (2023)
-
Hypothalamic control of energy expenditure and thermogenesis
Experimental & Molecular Medicine (2022)