Abstract
Doped Mott insulators are the starting point for interesting physics such as high temperature superconductivity and quantum spin liquids. For multi-band Mott insulators, orbital selective ground states have been envisioned. However, orbital selective metals and Mott insulators have been difficult to realize experimentally. Here we demonstrate by photoemission spectroscopy how Ca2RuO4, upon alkali-metal surface doping, develops a single-band metal skin. Our dynamical mean field theory calculations reveal that homogeneous electron doping of Ca2RuO4 results in a multi-band metal. All together, our results provide evidence for an orbital-selective Mott insulator breakdown, which is unachievable via simple electron doping. Supported by a cluster model and cluster perturbation theory calculations, we demonstrate a type of skin metal-insulator transition induced by surface dopants that orbital-selectively hybridize with the bulk Mott state and in turn produce coherent in-gap states.
Similar content being viewed by others
Introduction
Interface metallicity paves the way for two-dimensional fermionic gasses with interesting properties such as superconductivity1,2,3. Insulators are building blocks for such interfaces. Upon doping (charge transfer), two-dimensional metals can occur at the interface between two insulators like LaAlO3 and SrTiO34,5 or at the insulator-vacuum interface like the surface of SrTiO36,7. Such metallic states confined at interfaces are broadly dubbed quantum well states. This term is used irrespectively of insulating nature (band or Mott insulators). For many spectroscopies, insulator–vacuum interfaces are interesting as they are directly accessible in contrast to buried8 insulator-insulator quantum wells. Doping of insulator–vacuum interfaces are often achieved by dosing with alkali metal atoms9,10,11,12,13. Electrons from the alkali-metal layer can form a quantum-well state confined by vacuum and the band gap of the substrate. When the substrate band gap is sufficiently large, the metallic state can be strictly confined inside the alkali-metal overlayer11. On the other hand, with significant energy and momentum overlap, the quantum well state will hybridize with the substrate and form a hybrid state10,12. In this context, particularly interesting is the case of Mott insulators as a substrate. In contrast to the rigid band gap of band insulators, a Mott gap is maintained by a delicate balance between kinetic energy and electron correlation14. This in turn suggests that the interaction between the alkali metal and the electronic states of the Mott insulator could trigger the breakdown of the Mott state at the surface, leaving a hybrid quantum-well state.
Here we demonstrate—using photoemission spectroscopy—that alkali add-on atoms on Mott insulating Ca2RuO4 generates such a hybrid quantum well state. Independent of the chosen alkali-metal element (K, Rb, Cs), we observe a depletion of the lower-Hubbard-band (LHB) spectral weight and an evolution of the Ru core-level states as a function of doping, which are typical manifestations of a Mott breakdown15,16,17. Eventually, a single-band metal emerges as a result of the interaction between the alkali-metal dopants and the Ca2RuO4 substrate. Our work reveals a type of orbital-selective surface metal-insulator transition induced through in-gap state formation generated by orbital hybridization between surface dopants and the Mott insulating substrate (schematically illustrated in Fig. 1).
a Schematic representation of the Ca2RuO4 band-Mott insulating structure45. LHB and UHB denote the lower and upper Hubbard bands, respectively. In the short c-axis phase, the dxy band is fully occupied whereas the half-filled dxz/dyz orbitals are Mott insulating forming LHB and UHB. b Homogeneous bulk doping, and chemical or applied pressure are expected to generate a multi-band metal that involves all the t2g orbitals. c Impurity electron doping, from surface alkali-metal deposition, turns out to generate in-gap states through hybridization with the Mott insulating dxz/dyz orbitals. The in-gap states emerge in conjunction with partial spectral weight suppression of the Hubbard bands (indicated by dashed gray lines). d Re-metallization upon crossing a certain threshold of impurity doping level. Due to the orbital-selective hybridization between surface dopants and the high-energy incoherent excitations, a single-band metal is formed.
Results
Electronic-structure evolution by alkali-metal dosing
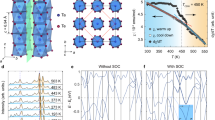
Rb 4p core level spectroscopy and electronic band structure of Ca2RuO4 single crystals as a function of alkali-metal deposition are shown in Fig. 2a and b, respectively. Angle-resolved photoemission spectroscopy (ARPES) spectra are recorded along the Γ–M direction. Before alkali-metal deposition, the electronic structure—shown in Fig. 2b, left panel – is consistent with previous reports15,18. Around the Brillouin zone center, dispersive bands (2.5–0.5 eV) are observed and the non-dispersive LHB is located about ~ 1.7 eV below the Fermi level (EF). Previously, the dispersive bands have been assigned predominately to the dxy orbital and the LHB to the dxz/dyz orbitals15,18,19. Upon dosing Rb, the whole structure is shifted downwards [Fig. 2b] and a Rb 4p core level peak develops [Fig. 2a]. By further dosing Rb, an in-gap state with an electron-like dispersion evolves from its band bottom. Whereas the low-energy spectral weight is initially negligible, the band extends with the increase of the Rb amount and finally produces finite spectral weight at EF (See Supplementary Fig. 1). These changes occur qualitatively in the same manner irrespective of the choice of alkali-metal dopants—see Fig. 2c, d for the case of K and Cs dosing.
a, b Rb 4p energy distribution curves (EDCs) and valence-band energy distribution maps (EDMs), respectively, recorded at the temperature of T = 150 K with dosing Rb in incremental steps. p-polarized 125-eV incident light is used. The momentum cut is indicated in the inset of the leftmost panel. The color bar quantifies photoemission intensity. c, d EDMs recorded with dosing K and Cs using p-polarized light of hν = 31 and 107 eV, respectively. Similar changes as the Rb case are observed.
Surface Mott breakdown in Ca2RuO4
Momentum-integrated energy distribution curves (EDCs) are plotted in Fig. 3a. Besides Ru 4d-derived states including LHB, O 2p states are identified at ~3.2 eV below EF18. Upon Rb dosing, the O 2p peak moves to higher binding energy until the shift saturates at ~0.3 eV, followed by a slight shift backward. While Ru ions could change their valency when doped with electrons, oxygen ions should remain chemically unperturbed. The O 2p peak position thus serves as a measure of the chemical-potential shift as demonstrated by previous studies of oxide materials16,20. In Fig. 3b, we align the O 2p peak position to compensate for the chemical-potential shift and unravel, in this fashion the intrinsic lower-energy structures. The total spectral intensities, within the displayed energy window, are normalized to eliminate attenuation effects from the Rb overlayer. After these data treatments, a prominent decay of LHB intensity (magenta-shaded region) is observed concomitantly with the growth of near-EF spectral weight (green-shaded region)—see Fig. 3c. In contrast to the drastic changes in the spectral weight, the position of the LHB is essentially independent of alkali dosing [Fig. 3d]. This suggests that the size of the Mott gap is unaffected by alkali-metal dosing even though the chemical potential moves inside the gap. Instead of the shrinkage of the gap size, the collapse of the Mott gap is driven by the spectral-weight loss in the Hubbard bands.
a Valence-band energy distribution curves (EDCs) versus energy relative to EF plotted in the order of Rb deposition levels. Spectra are given arbitrary vertical offsets for a visibility purpose. The inset indicates the momentum window for integration. b Valence band EDCs aligned to the O 2p peak position and normalized to the total intensity in the displayed energy region. c Spectral weight of the lower Hubbard band (LHB) and the near-EF part integrated within the magenta and green shaded regions, respectively, in (b). The LHB spectral weight is estimated after subtracting a tangential linear background shown by dotted lines in (b). The weight has been normalized to the maximum values. d The position of LHB with respect to the O 2p peak position plotted as a function of the Rb deposition sequence. The error bar is determined from intensity variation around the peak surpassing the noise level.
Core-level structure
The Ru 3d core levels, probed by x-ray photoemission spectroscopy (XPS), provide insights into the dosing-induced metallic surface state. In Mott insulating Ca2RuO4 and Y2Ru2O7, the Ru 3d peak is composed of a single set of spin-orbit-split peaks (3d5/2 and 3d3/2) as shown in Fig. 4a, b. Isovalent Bi substitution for Y17 drives a band-width-controlled Mott transition, and the resulting metallic state of Bi2Ru2O7 yields splittings within the 3d5/2 and 3d3/2 levels [Fig. 4b]. It has been proposed that the low-energy peak stems from a final state where core holes are screened by conduction electrons17. Upon Cs dosing of Ca2RuO4, a very similar splitting of the core levels is observed [Fig. 4a]—suggesting the emergence of conduction electrons with Ru character.
a Ru 3d spectra of Ca2RuO4 measured by x-ray photoemission spectroscopy (XPS) before and after dosing Cs at 300 K. b Ru 3d XPS spectra of Mott-insulating Y2Ru2O7 and metallic Bi2Ru2O7 from ref. 17 with the binding energy aligned to that of Ca2RuO4.
Surface metallic state
Having tracked in detail the evolution of the electronic structure by alkali-metal dosing, we now focus on the character of the created metallic state. To embed the momentum-resolved photoemission signal into the Brillouin zone of Ca2RuO4, we use the constant-energy map intersecting oxygen bands18—see Fig. 5a. Then, Fermi surface after metallization is mapped out in the same momentum regions. The resulting map [Fig. 5b] reveals circular Fermi surfaces in accordance with the tetragonal Brillouin zone. The bulk crystal structure of Ca2RuO4 is orthorhombic. Numerous ARPES studies have accumulated evidence that the potential of orthorhombic distortion is strong enough to cause band folding21,22,23,24,25. The absence of band folding suggests that the metallic surface state is not strictly confined in the Ca2RuO4 crystal. The spectral intensity of the electron-like band composing the Fermi surface depends strongly on incident light polarization [Fig. 5c–e] and is suppressed with s-polarized light. In contrast, the band is visible with p-polarized light irrespective of the azimuthal angle of the mirror plane [see also Fig. 2b, c]. The band therefore possesses in-plane even character. As shown in Fig. 5f, the metallic band exhibits a kink (sudden change of band velocity) at ~ 0.4 eV below EF (see also Supplementary Fig. 2 for detailed analysis). This is also evidenced by a saturation of the momentum distribution curve (MDC) linewidth [Fig. 5g].
a Constant-energy map of pristine Ca2RuO4 at E = EF − 5.1 eV recorded with hν = 90 eV, p-polarized photons at the temperature of T = 150 K. The oxygen bands draw a periodic structure. Overlaid gray solid and dashed squares represent Brillouin zones in the tetragonal and orthorhombic notations, respectively. b Fermi surface after K dosing for 10 min at 6.5 A. The color bar quantifies photoemission intensity. c–e Energy distribution maps (EDMs) recorded with circular, p, and s polarization, respectively, along the cut indicated in (b). The band becomes indiscernible when measured with s-polarized light. f EDM of the K-dosed state [identical to K-3 in Fig. 2c] overlaid with momentum-distribution-curve (MDC) peak positions, recorded with hν = 31 eV, p-polarized photons along the cut shown in the inset. g MDC full width at half maximum averaged over the two branches. The dashed curve indicates the low-energy dependence to guide the eye. Kinks in the MDC peak position and width are indicated by arrows. Error bars are based on the standard deviation of the fitting.
Discussion
Our main observation is a metallization of Ca2RuO4 upon application of alkali-metal atoms. A central question is whether this quantum well state is a hybrid state between alkali metals and Ca2RuO4. We address this question by inspecting: (i) the spectral weight of the LHB, (ii) the Ru-core levels, and (iii) the self-energy effects of the induced quantum well state.
(i) Mono-, bi-, and tri-layer alkali-metal deposition have been reported9,26 on Sr2IrO4 and Sr2RuO4. Due to the large inelastic mean free path of alkali metals27, bulk bands are observed through the alkali-metal layers in both cases. The observation of drastic LHB suppression (Fig. 3) is thus intrinsic and not an artifact of an alkali-metal overlayer. With few exceptions28, spectral weight suppression of the Hubbard bands is associated with quasi-particle formation near the chemical potential16,29. This is consistent with our observation of a fading Hubbard band being replaced by a valence band as a function of alkali-metal dosing.
(ii) Also the Ru core level [Fig. 4a] is modified by alkali-metal deposition. The simplest possibility of lower-energy satellite in the Ru 3d peak is a Ru3+ component created by doping Ru4+ with an electron. However, the intense low-energy peak translates into more than 0.6 electrons doped per Ru atom. This value is unrealistic as previous alkali-metal adsorption studies on oxides found at most ~0.15 electrons doped per atom9,13,16,26. Instead, such an intense low-energy satellite peak, which appears upon metallization of Mott insulators like cuprates30,31 and ruthenates17,32, has been associated with a final state where core holes are efficiently screened by conduction electrons. The change induced by alkali-metal dosing [Fig. 4a] thus suggests the emergence of itinerant electrons with, at least partially, the Ru 3d character. We note that the linewidth of other core levels such as Ca 3p is essentially insensitive to dosing of alkali atoms [see Supplementary Fig. 3] suggesting that the Ru 3d transformation is intrinsic.
(iii) Finally, the kink witnessed both in the band dispersion [Fig. 5f] and MDC linewidth [Fig. 5g] of the metallic band suggests strong self-energy effects. The energy scale of 0.4 eV is incompatible with electron-phonon interactions and rather points to electron-electron interactions. In fact, such high-energy kinks have been widely observed in strongly correlated systems like cuprates33,34 and ruthenates35, and interpreted as a manifestation of many-body self-energy effects. The kink, therefore, suggests substantial electron correlations induced by substrate Ru orbitals. Such correlation effects are not expected (or reported) for a purely alkali-metal quantum-well states11.
Based on observations (i)–(iii), we conclude that the induced metallic state involves an interaction between alkali-metal atoms and Ca2RuO4.
So far, bulk electron doping of Ca2RuO4 has been achieved only by substituting La or Pr for Ca36,37,38. This substitution inevitably involves chemical pressure. As a similar metal-insulator transition is observed with isovalent Sr substitutions39, chemical pressure rather than doping is most likely the metallization mechanism of La/Pr doping. In all cases, the resulting metallic state comprises multiple Fermi surfaces—composed of almost evenly filled dxz, dyz, and dxy orbitals15,24. These electronic structures are realistically captured by dynamical mean-fied theory (DMFT) calculations, which take into account both electronic structure and strong correlations (self-energy) effects15,24. In the present surface-dosed case, it is unclear whether the transition involves c-axis changes. We therefore performed DMFT calculations for the short c-axis phase to evaluate genuine electron-doping effects. As shown in Supplementary Fig. 4, electron doping again leads to the formation of multiple Fermi surface sheets (see also Supplementary Note 1). This demonstrates that alkali metal dosing does not correspond to the standard theoretical description of homogeneous electron doping [Fig. 1b]. Surface re-metallization due to Coulomb screening is also expected to yield a multiband metal.
Inspection of the initial stage of the band-structure evolution by alkali-metal dosing [Fig. 2b–d] indicates no detectable spectral weight accumulation at EF. This is in direct contrast to electron doping of Mott insulating cuprates16,40, and is an indication that the charge transfer of the alkali valence electrons to the upper Hubbard band (UHB) is not complete. This result suggests a covalent nature of bonding with partial charge transfer to the Ru bands. Alkali-metal s electrons are thus not directly injected into the UHB. Instead, they reside on localized impurity states with pinned chemical potential within the gapped region. Such impurity states can hybridize with the Ru d orbitals provided sufficient spacial overlap and symmetry compatibility. Linear combination of dxz and dyz orbitals (with even parity) is the most obvious candidate for such hybridization. The formation of interfacial bonding states between surface-dosed alkali-metal s and transition-metal d orbitals has been frequent observation in previous photoemission studies12,41,42,43,44.
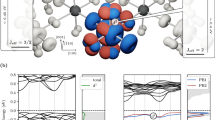
We therefore evaluated the orbital-dependent spectral function via exact diagonalization of a cluster made of one Ru site and one impurity level. The model consists of the local interaction terms at the Ru and the impurity sites, and the kinetic term t among the dxz/dyz and the impurity orbitals. The following choices are made for the parameters: U = 2 eV, J = 0.5 eV, ΔCF = 0.3 eV, λ = 0.075 eV, UI = 1 eV18,45,46, where U is the Coulomb interaction between Ru 4d electrons, J is the Hund’s coupling, ΔCF is crystal-field splitting within the t2g sector, λ is spin-orbit coupling, and UI is the Coulomb interaction within the impurity level. We assume that the impurity level lies close to the bottom of the UHB and we mimic the increase of the doping concentration by increasing the parameter t, allowing more hybridization between the Ru dxz/dyz and impurity level. As shown in Fig. 6a, b, the initial change with small t is the shift of chemical potential to accommodate the impurity level. Upon increasing hybridization, the spectral weight of the dxz/dyz LHB located at E ~ EF − 2 eV is significantly suppressed and instead a new state with a mixed character of Ru dxz/dyz and impurity s appears near EF [Fig. 6c]. Further increase of t results in accumulating the spectral weight of this bonding state [Fig. 6d]. The overall changes are in good agreement with the experiment. We can provide a qualitative interpretation of the cluster calculations by analyzing the multiplet eigenstates and electronic transitions for the Ru and the alkali impurity state at the ionic level. A detailed analysis is reported in Supplementary Figs. 5, 6, Supplementary Notes 2 and 3. The ground state is built up as a quantum superposition of local \(\left\vert d,s\right\rangle\) configurations with a fully occupied dxy orbital, due to the extreme flattening of the Ru-O octahedra. Assuming a selective hybridization among the s level and dxz/dyz doublet, this superposition involves \(\left\vert {d}^{4},{s}^{1}\right\rangle\) and \(\left\vert {d}^{5},\underline{s}\right\rangle\) states. Thus, two spectral features can be obtained via the removal of one electron from the dxz/dyz doublet, which arise from an electronic transition to the \(\left\vert {d}^{3}\,{s}^{1}\right\rangle\) and \(\left\vert {d}^{4},\underline{s}\right\rangle\) state, respectively. While the former contributes to the weight of the LHB, the latter corresponds to the in-gap states and is expected to increase its spectral weight as the deposition sequence goes on.
a–d Spectral function calculated for Ru ion, t = 0.01 eV, t = 0.1 eV, and t = 0.2 eV, respectively, where t represents the hybridization term. Superimposed are energy distribution maps from Fig. 2b with Rb deposition sequences as indicated. The color bar quantifies photoemission intensity.
The effects of such orbital-selective hybridization mechanism on the extended system are schematized in Fig. 1d. Here we show that the Mott breakdown takes place due to the progressive depletion of the dxz/dyz LHB and consequent filling of the in gap impurity-driven states. It is worthwhile to notice that such mechanism has side effects also on the dxy band through Hund’s coupling. Due to the covalent nature of the ground state, the spectral weight associated to the dxy removal states spreads out between several allowed electronic transitions at slightly higher binding energies (See Supplementary Figs. 5, 6, and Supplementary Note 3 for details), depleting the dxy spectral weight in the low energy state (Fig. 6c) as seen experimentally (Fig. 2). After sufficient dosing (Rb-8 in Fig. 2), ARPES reveals broad spectral weight around the binding energy of 0.5–1 eV, spreading over a wide momentum range. Referring to the theoretical prediction (Fig. 6d), this electronic state is likely of dxy origin.
Finally, we notice that the orbital-selective hybridization can naturally lead to the formation of a single-sheet Fermi surface that is not reproduced by the homogeneous doping picture. The symmetry analysis of the metallic state (Fig. 5) is also compatible with the in-plane even character of the bonding state. To further explore compatibility with the observation of a single Fermi surface sheet with free-electron-like dispersion, we combined the cluster calculation with cluster perturbation theory (CPT). The latter uses exact diagonalization of small clusters to construct a strong-coupling perturbation theory for the lattice problem47 (see Supplementary Note 4). Even though this approach represents a simplification of the complex physics characterizing the large dopant regime, it allows to determine the Fermi surfaces to be compared to our experimental results. In particular, we are interested in the evolution with the alkali-metal content of the low-energy features corresponding to the in-gap states. In the atomic regime, as demonstrated in the cluster calculation, the in-gap localized states are mainly made of s- and dxz/dyz states with a relative charge distribution dictated by the hybridization and the multiplet configurations at the Ru sites. These localized states can overlap along the Ru-O-Ru bond directions. In order to understand the formation of the Fermi pocket as due to the hybridization of the s-states with the Ru d-bands, it is particularly instructive to consider the limit with no direct overlap between the s-states. In this case, the effective mass of the s-state is due to the hybridization through the mixing with the Ru d-states across the Mott gap. As shown in Fig. 7a, b, we observe that the impurity level acquires an effective mass with a dispersion that substantially follows that of the dxz/dyz bands, due to the local hybridization. Since the dxz and dyz states have a quasi 1D electronic dispersion of the type coskx and cosky, respectively, electrons in the s-state can propagate both along the x and y directions in the lattice. As a consequence of the orbital hybridization, the effective acquired dispersion of the s-state will have the following general structure \({\epsilon }_{s}({k}_{x},{k}_{y})=-2\tilde{t}(\cos {k}_{x}+\cos {k}_{y})\). Here, due to the correlated nature of the hosting electronic states, the effective hopping amplitude \(\tilde{t}\) depends on the Coulomb interaction and the spin-orbit coupling at the Ru site as well as on the electron density of the impurity level. Taking into account the form ϵs(kx, ky) of the dispersion close to the Fermi energy, the resulting Fermi pocket is isotropic as shown in Fig. 7c, d. Due to the hybridization among the impurity s- and the Ru dxz/dyz states, the emerging Fermi pocket for the s-state has also a non-vanishing spectral weight projected on the dxz and dyz orbital configurations. The displayed Fermi surface for the concomitant projection on both dxz and dyz [Fig. 7c] thus has the same profile as that for the s-states, even though the projection on the single orbital configuration (dxz or dyz) would yield a Fermi line that is anisotropic and reflects the dominant 1D like character of the dxz and dyz bands. This result is confirming that our orbital selective model supports the formation of a single-sheet isotropic Fermi pocket.
Electronic dispersions yielded by cluster perturbation theory calculations at ky = 0 by varying kx in the range [−π, π] for the bands (a) arising from the Ru dxz/dyz orbitals and (b) from the impurity s-state at the alkali site. Black lines identify the Fermi level EF. The color bar quantifies the spectral function. Fermi pocket of the (c) Ru dxz/dyz bands and (d) impurity band. The corresponding Fermi lines indicate the occurrence of an electron pocket centered at Γ. The spectral weight of the pocket is made of hybridized dxz/dyz and s bands. The computation refers to an electronic configuration with vanishing direct hybridization between the impurity states on nearest neighbors. The dispersion of the impurity s-state is yielded through the local hybridization with the Ru d-orbitals and the Ru-Ru effective charge transfer.
The analysis has been performed for a representative case for the set of parameters used for the single site cluster calculation. Small variations do not affect the qualitative character of the Fermi pocket. The overall outcome is compatible with our experimental observation. We conclude that this scenario provides a novel type of surface Mott-insulator to metal transition realized through chemical doping. Note that a metallic in-gap state can also emerge by directly injecting carriers to the Hubbard bands16,48. However, the present single-band in-gap state is incompatible with such direct carrier doping and instead suggests the orbital-selective formation of covalent bonds.
Due to the reduced coordination at surfaces, correlated systems have the opposite tendency, namely that of spontaneously forming a skin with suppressed conductivity. This has been observed and discussed in various oxides such as vanadates49,50,51, cuprates30, and ruthenates52,53. The present work demonstrates the opposite case where dosed alkali metals increase surface hopping channels and produces a metallic skin on the Ca2RuO4 Mott insulating state. Although the multiband Mott insulating state of Ca2RuO4 is rather unique in nature, similar physics could be realized in other systems. Typical ingredients would be quasi-two-dimensional materials with Mott bands composed of inter-layer directed orbitals (pz, dz or dxz) and moderate electron correlation. These criteria are uniquely satisfied in Ca2RuO4. The Mott bands have predominately 4dxz/yz character and the moderate correlation strength makes hybridization with deposited alkali-metal electrons possible.
Methods
Sample preparation and photoemission experiments
High-quality Ca2RuO4 single crystals were grown by the flux-feeding floating-zone method54,55. ARPES measurements were carried out at the MAESTRO and I05 beamlines at Advanced Light Source and Diamond Light Source, respectively, at 150 K in the insulating short c-axis phase. Photon energy was varied in the range of 30–130 eV with energy resolution better than 50 meV. Presented ARPES data are recorded either using s-, p-, or circularly polarized light, with s and p denoting odd and even parity with respect to the photoemission mirror plane, respectively. XPS measurements were conducted at BL07LSU of SPring-8 at 300 K and 500 eV of incident photon energy. Samples #1–#3 and #5 were measured with ARPES and sample #4 with XPS. For all the photoemission measurements, samples were cleaved in situ using the top-post method. SAES Getter dispensers were used to evaporate K, Rb, or Cs onto the Ca2RuO4 surface in incremental steps. Unless otherwise stated, one dose corresponds to evaporation of K, Rb, and Cs for respectively 40, 30, and 30 s with a filament current of 6.6, 6.4, and 8.3 Ampere. Note that Cs dosing for the ARPES and XPS measurements were done at different instruments and hence the dose unit is not exactly equivalent. No detectable charging was observed when varying the photon flux as long as the temperature was kept above 150 K. The tetragonal notation with a = 3.80 Å is used to display ARPES data.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Caviglia, A. D. et al. Electric field control of the LaAlO3/SrTiO3 interface ground state. Nature 456, 624–627 (2008).
Kim, Y. K., Sung, N. H., Denlinger, J. D. & Kim, B. J. Observation of a d-wave gap in electron-doped Sr2IrO4. Nat. Phys. 12, 37–41 (2016).
Liu, C. et al. Two-dimensional superconductivity and anisotropic transport at KTaO3 (111) interfaces. Science 371, 716–721 (2021).
Liu, C. et al. Tunable superconductivity and its origin at KTaO3 interfaces. Nat. Commun. 14 https://doi.org/10.1038/s41467-023-36309-2 (2023).
Reyren, N. et al. Superconducting interfaces between insulating oxides. Science 317, 1196–1199 (2007).
Santander-Syro, A. F. et al. Two-dimensional electron gas with universal subbands at the surface of SrTiO3. Nature 469, 189–193 (2011).
Moser, S. et al. Tunable polaronic conduction in anatase TiO2. Phys. Rev. Lett. 110, 196403 (2013).
Woerle, J. et al. Electronic band structure of the buried SiO2/SiC interface investigated by soft x-ray ARPES. Appl. Phys. Lett. 110, 132101 (2017).
Kim, Y. K. et al. Fermi arcs in a doped pseudospin-1/2 Heisenberg antiferromagnet. Science 345, 187–190 (2014).
Alidoust, N. et al. Observation of monolayer valence band spin-orbit effect and induced quantum well states in MoX2. Nat. Commun. 5, 4673 (2014).
Eknapakul, T. et al. Nearly-free-electron system of monolayer Na on the surface of single-crystal HfSe2. Phys. Rev. B 94, 201121 (2016).
Akikubo, K. et al. Observation of an eg-derived metallic band at the Cs/SrTiO3 interface by polarization-dependent photoemission spectroscopy. Thin Solid Films 603, 149–153 (2016).
Yukawa, R. et al. Control of two-dimensional electronic states at anatase TiO2(001) surface by K adsorption. Phys. Rev. B 97, 165428 (2018).
Imada, M., Fujimori, A. & Tokura, Y. Metal-insulator transitions. Rev. Mod. Phys. 70, 1039–1263 (1998).
Riccò, S. et al. In situ strain tuning of the metal-insulator-transition of Ca2RuO4 in angle-resolved photoemission experiments. Nat. Commun. 9, 4535 (2018).
Hu, C. et al. Momentum-resolved visualization of electronic evolution in doping a Mott insulator. Nat. Commun. 12, 1356 (2021).
Kim, Hyeong-Do, Noh, Han-Jin, Kim, K. H. & Oh, S.-J. Core-Level X-ray photoemission satellites in ruthenates: a new mechanism revealing the Mott transition. Phys. Rev. Lett. 93, 126404 (2004).
Sutter, D. et al. Hallmarks of Hunds coupling in the Mott insulator Ca2RuO4. Nat. Commun. 8, 15176 (2017).
Curcio, D. et al. Current-driven insulator-to-metal transition without Mott breakdown in Ca2RuO4. Phys. Rev. B. 108 https://doi.org/10.1103/PhysRevB.108.L161105 (2023).
Shen, K. M. et al. Missing quasiparticles and the chemical potential puzzle in the doping evolution of the cuprate superconductors. Phys. Rev. Lett. 93, 267002 (2004).
Damascelli, A. et al. Fermi surface, surface states, and surface reconstruction in Sr2RuO4. Phys. Rev. Lett. 85, 5194–5197 (2000).
Tamai, A. et al. Fermi surface and van Hove singularities in the itinerant metamagnet Sr3Ru2O7. Phys. Rev. Lett. 101, 026407 (2008).
Liu, Y., Nair, H. P., Ruf, J. P., Schlom, D. G. & Shen, K. M. Revealing the hidden heavy Fermi liquid in CaRuO3. Phys. Rev. B 98, 041110 (2018).
Sutter, D. et al. Orbitally selective breakdown of Fermi liquid quasiparticles in Ca1.8Sr0.2RuO4. Phys. Rev. B 99, 121115 (2019).
Horio, M. et al. Electronic reconstruction forming a C2-symmetric Dirac semimetal in Ca3Ru2O7. npj Quantum Mater. 6, 29 (2021).
Kyung, W. et al. Electric-field-driven octahedral rotation in perovskite. npj Quantum Mater. 6, 5 (2021).
Smith, N. V., Wertheim, G. K., Andrews, A. B. & Chen, C.-T. Inelastic electron mean free paths in the alkali metals: effect of the empty d bands. Surf. Sci. 282, L359–L363 (1993).
Zhou, X. et al. Angle-resolved photoemission study of the Kitaev candidate α−RuCl3. Phys. Rev. B 94, 161106 (2016).
Eskes, H., Meinders, M. B. J. & Sawatzky, G. A. Anomalous transfer of spectral weight in doped strongly correlated systems. Phys. Rev. Lett. 67, 1035–1038 (1991).
Taguchi, M. et al. Evidence for suppressed screening on the surface of high-temperature La2−xSrxCuO4 and Nd2−xCexCuO4 superconductors. Phys. Rev. Lett. 95, 177002 (2005).
Horio, M. et al. Electronic structure of Ce-doped and -undoped Nd2CuO4 superconducting thin films studied by hard X-ray photoemission and soft X-ray absorption spectroscopy. Phys. Rev. Lett. 120, 257001 (2018).
Cox, P. A., Egdell, R. G., Goodenough, J. B., Hamnett, A. & Naish, C. C. The metal-to-semiconductor transition in ternary ruthenium (IV) oxides: a study by electron spectroscopy. J. Phys. C: Solid State Phys. 16, 6221–6239 (1983).
Xie, B. P. et al. High-energy scale revival and giant kink in the dispersion of a cuprate superconductor. Phys. Rev. Lett. 98, 147001 (2007).
Chang, J. et al. When low- and high-energy electronic responses meet in cuprate superconductors. Phys. Rev. B 75, 224508 (2007).
Iwasawa, H. et al. High-energy anomaly in the band dispersion of the ruthenate superconductor. Phys. Rev. Lett. 109, 066404 (2012).
Fukazawa, H. & Maeno, Y. Filling control of the Mott insulator Ca2RuO4. J. Phys. Soc. Jpn. 70, 460–467 (2001).
Cao, G. et al. Ground-state instability of the Mott insulator Ca2RuO4: impact of slight La doping on the metal-insulator transition and magnetic ordering. Phys. Rev. B 61, R5053–R5057 (2000).
Pincini, D. et al. Tuning of the Ru4+ ground-state orbital population in the 4d4 Mott insulator Ca2RuO4 achieved by La doping. Phys. Rev. B 99, 075125 (2019).
Nakatsuji, S. & Maeno, Y. Quasi-two-dimensional Mott transition system Ca2−xSrxRuO4. Phys. Rev. Lett. 84, 2666–2669 (2000).
Armitage, N. P. et al. Doping dependence of an n-type cuprate superconductor investigated by angle-resolved photoemission spectroscopy. Phys. Rev. Lett. 88, 257001 (2002).
Soukiassian, P. et al. Adsorbate-induced shifts of electronic surface states: Cs on the (100) faces of tungsten, molybdenum, and tantalum. Phys. Rev. B 31, 4911–4923 (1985).
Ozawa, K. et al. Potassium adsorption on the polar NbC(111) surface: angle-resolved photoemission study. Surf. Sci. 375, 250–256 (1997).
Ozawa, K. et al. Photoemission study of K adsorption on ZrC(111). Surf. Sci. 433-435, 700–704 (1999).
Zhang, S. et al. Time-resolved photoemission study of the electronic structure and dynamics of chemisorbed alkali atoms on Ru(0001). Phys. Rev. B 93, 045401 (2016).
Das, L. et al. Spin-orbital excitations in Ca2RuO4 revealed by resonant inelastic X-ray scattering. Phys. Rev. X 8, 011048 (2018).
Gauquelin, N. et al. Pattern Formation by Electric-Field Quench in a Mott Crystal. Nano Lett. 23, 7782–7789 (2023).
Sénéchal, D., Perez, D. & Plouffe, D. Cluster perturbation theory for hubbard models. Phys. Rev. B 66, 075129 (2002).
Dagotto, E., Ortolani, F. & Scalapino, D. Single-particle spectral weight of a two-dimensional hubbard model. Phys. Rev. B 46, 3183–3186 (1992).
Sekiyama, A. et al. Mutual experimental and theoretical validation of bulk photoemission spectra of Sr1−xCaxVO3. Phys. Rev. Lett. 93, 156402 (2004).
Rodolakis, F. et al. Quasiparticles at the Mott transition in V2O3: wave vector dependence and surface attenuation. Phys. Rev. Lett. 102, 066805 (2009).
Yoshimatsu, K. et al. Dimensional-crossover-driven metal-insulator transition in SrVO3 ultrathin films. Phys. Rev. Lett. 104, 147601 (2010).
Takizawa, M. et al. Manifestation of correlation effects in the photoemission spectra of Ca1−xSrxRuO3. Phys. Rev. B 72, 060404 (2005).
Panaccione, G. et al. Depth dependence of itinerant character in Mn-substituted Sr3Ru2O7. N. J. Phys. 13, 053059 (2011).
Fukazawa, H., Nakatsuji, S. & Maeno, Y. Intrinsic properties of the Mott insulator Ca2RuO4+δ (δ = 0) studied with single crystals. Phys. B Condens. Matter 281, 613–614 (2000).
Nakatsuji, S. & Maeno, Y. Synthesis and single-crystal growth of Ca2−xSrxRuO4. J. Solid State Chem. 156, 26 – 31 (2001).
Acknowledgements
Fruitful discussion with M. Grioni is greatfully acknowledged. M. Horio, D.S., C.G.F., C.E.M., and J.C. acknowledge support by the Swiss National Science Foundation under grant no. 200021_188564. M. Horio and T.W. are supported by Grants-in-aid from the Japan Society of the Promotion of Science (JSPS) (grant No. 21K13872). M.K. was supported by KIAS Individual Grants (CG083501). This work was supported in part by the Italian Ministry of Foreign Affairs and International Cooperation, grant number KR23GR06. S.M. acknowledges support from the Swiss National Science Foundation under grant no. P300P2-171221. Y.S. is funded by the Swedish Research Council (VR) with a Starting Grant (Dnr. 2017-05078). G.S. acknowledges the hospitality of the Center for Computational Quantum Physics at the Flatiron Institute, a division of the Simons Foundation. G.S. and S.M. acknowledge funding support from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany ’s Excellence Strategy through the Würzburg-Dresden Cluster of Excellence on Complexity and Topology in Quantum Matter ct.qmat (EXC 2147, Project ID 390858490) as well as through the Collaborative Research Center SFB 1170 ToCoTronics (Project ID 258499086). This research used resources from the Advanced Light Source, which is a DOE Office of Science User Facility under contract no. DE-AC02-05CH11231. We acknowledge Diamond Light Source for time at beamline I05 under proposal SI10550. We are grateful to the CPHT computer support team for the DFT +DMFT computation.
Author information
Authors and Affiliations
Contributions
V.G., R.F., and A.V. grew the Ca2RuO4 single crystals. J.C. and H.M.R. conceived the ARPES project. M. Horio, D.S., C.G.F., C.E.M., S.M., Y.S., G.G., and J.C. carried out the ARPES experiments. The ARPES data were analyzed by M. Horio and D.S. M. Horio and T.W. conceived and performed the XPS experiments and analyzed the data. Photoemission beamlines were developed and maintained by M. Hoesch, T.K.K., S.M., C.J., A.B., E.R., and I.M. DMFT calculations were conducted by M.K., A.G., and G.S. Cluster-diagonalization calculations were carried out by F.F. and M.C. M.Horio, F.F., M.C., and J.C. wrote the manuscript with inputs from other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Physics thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Horio, M., Forte, F., Sutter, D. et al. Orbital-selective metal skin induced by alkali-metal-dosing Mott-insulating Ca2RuO4. Commun Phys 6, 323 (2023). https://doi.org/10.1038/s42005-023-01436-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42005-023-01436-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.