Abstract
Plasma-activated chemical transformations promise the efficient synthesis of salient chemical products. However, the reaction pathways that lead to desirable products are often unknown, and key quantum-state-resolved information regarding the involved molecular species is lacking. Here we use quantum cascade laser dual-comb spectroscopy (QCL-DCS) to probe plasma-activated NH3 generation with rotational and vibrational state resolution, quantifying state-specific number densities via broadband spectral analysis. The measurements reveal unique translational, rotational and vibrational temperatures for NH3 products, indicative of a highly reactive, non-thermal environment. Ultimately, we postulate on the energy transfer mechanisms that explain trends in temperatures and number densities observed for NH3 generated in low-pressure nitrogen-hydrogen (N2–H2) plasmas.
Similar content being viewed by others
Introduction
Through its use in industrial fertilizers1, ammonia (NH3) makes an indispensable contribution to global agriculture by helping to feed about 50% of the world’s population2. Because of its critical place within our food supply chain, current research into plasma-activated NH3 formation aims to reduce costs, improve distribution and increase efficiency as compared to the energy-intensive and ubiquitous Haber–Bosch process3. Ideally, plasma activated NH3 production would be achieved by nitrogen (N2) fixation from air, preferably at lower temperatures and pressures than are currently required. However, despite significant efforts, the energy yields reported for plasma-activated NH3 formation remain more than one order-of-magnitude lower than that of the conventional Haber–Bosch process4. To overcome this deficit, it is crucial to improve our understanding of formation processes at the molecular level. Beyond its application to addressing the challenges of a global food supply chain, improved knowledge of plasma-activated NH3 formation mechanisms could also impact our future energy needs by improving mitigation strategies applied to gas reprocessing in tokamak fusion reactors5.
Because plasmas comprise high-energy electrons, ions, radicals and neutral molecules together in a confined environment, there exist myriad pathways that may lead to the breaking and formation of chemical bonds, and therefore a large number of possible chemical transformations. Generally, NH3 formation in plasma reactors is ascribed to the stepwise hydrogenation of adsorbed nitrogen atoms (N), imidogen radicals (NH) and amino radicals (NH2) found at various reactor surfaces6,7. If correct, this mechanism should yield NH3 molecules in non-thermal equilibrium, an environment characterized by different temperatures being ascribed to different degrees of freedom of the molecule (translational, rotational and vibrational). Consequently, molecules in non-thermal equilibrium can exhibit enhanced or reduced rate constants for state-specific, vibrationally mediated reactions8. Therefore, to better understand the pathways to plasma-activated NH3 formation, we require quantum-state-resolved information on the NH3 molecule in operando. Although we focus on NH3 in this work, quantum-state-resolved information and its impact on reaction pathways is of high importance to other plasma driven chemical syntheses9.
To date, several active laser diagnostics have been used to probe NH3 molecules in plasma reactors. They are cavity-enhanced absorption spectroscopy7,10, tuneable diode laser absorption spectroscopy7,11 and quantum-cascade laser absorption spectroscopy11,12,13. These techniques rely upon stepwise scanning or sweeping the wavelength of a continuous-wave (CW) laser to measure the molecular absorption. Only a few absorption features are generally accessible to any one CW laser, limiting the scope of their application to only a sub-set of the complex plasma processes that occur between species within a non-thermal environment. To increase the spectral coverage and hence the number of quantum states that can be probed via CW laser scanning, external cavity quantum-cascade lasers can be used to provide broader spectral coverage14,15.
Considering active laser diagnostics for plasmas more broadly, direct frequency-comb Fourier transform spectroscopy has been used to detect multiple species in the effluent of a dielectric barrier discharge16 and dual-comb spectroscopy has studied time-resolved spectra from a CH4/He electric discharge17. In the visible wavelength region, dual-comb spectroscopy has also been used to detect trace amounts of the atomic species Rb and K after ejection from a laser-induced breakdown of a solid target18. But no previous demonstrations of comb spectroscopy have revealed a quantum-state-resolved picture of non-thermal plasmas applied to grand societal challenges like N2 fixation to NH3.
Apart from molecular plasmas, non-thermal effects (i.e., non-local thermal equilibrium) are commonly reported in several extreme environments, including in laser-induced plasmas19, combustion environments20, and molecules relevant to the study of exoplanetary atmospheres21. Laser diagnostics have been applied as quantitative sensing tools across these extreme environments, including for the study of kinetics inside combustion chambers22, shock tubes, rapid compression machines, and flames.
Here, we apply quantum cascade laser dual-comb spectroscopy (QCL-DCS)23,24 at a wavelength near \(\lambda\) = 9.4 μm to study NH3 formation in a low-pressure, nitrogen-hydrogen (N2–H2) plasma. This study highlights several advantages of QCL-DCS as a diagnostic tool for probing complex environments like a non-thermal plasma, including high spectral resolution (4.5 × 10−4 cm−1 or 14 MHz) and broad spectral coverage (50 cm−1). Previously, QCL-DCS has been applied to jet-cooled molecular expansions25 and thermally populated gas samples at ambient temperature26 to demonstrate high-resolution, and to condensed-phase biological systems to demonstrate fast (μs) acquisition rates27. Here we combine these two inherent advantages—trading high time resolution for high frequency resolution via fast interleaving—to perform high-resolution molecular spectroscopy of a complex, non-thermal environment inside of a research-grade industrial plasma reactor.
Results
Plasma-activated NH3 generation probed by dual-comb spectroscopy
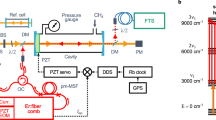
We probe plasma-activated NH3 generation via line-of-sight laser absorption spectroscopy in the long-wave infrared, near \(\widetilde{\nu }\) = 1060 cm−1 (\(\widetilde{\nu }=c/\lambda\) in units of cm−1 and c is the speed of light). A schematic of the experimental set-up is shown in Fig. 1a. Briefly, the output from the first QCL comb with a repetition rate, frep,1 = 7.417 GHz propagates along two distinct free-space beam paths, creating a probe beam and a reference beam. The probe beam is coupled to a multi-pass cell (path length of \(L\) = 3.16 m) attached to the plasma reactor, and the reference beam bypasses the reactor. A second local-oscillator QCL comb with slightly different repetition rate (frep,2 = frep,1 + Δfrep, where Δfrep = 2.1 MHz) is spatially overlapped with the probe and reference beams, respectively, at two different photodetectors, thus creating both probe and reference down-converted DCS signals (interferograms) for spectral analysis and optical power normalization. Additional details are provided in the Methods section.
a Schematic of the experimental setup. The probe beam from QCL comb 1 is coupled to a multi-pass cell attached to the plasma reactor, whereas the reference beam bypasses the reactor. Probe and reference combs are combined with copies of the local-oscillator QCL comb 2 at two different photodetectors. Dashed lines indicate electronic connections. Reactor temperatures \({T}_{{{{{{\rm{wall}}}}}}}\) and \({T}_{{{{{{\rm{load}}}}}}}\) were measured where indicated by the black arrows, and the non-thermal region (distance between the inner walls) of the reactor is indicated by the left-right arrow. b Energy level diagram29 for NH3 at the ν2 vibration, as probed by QCL-DCS (vertical arrows). The transition energies (in wavenumber) of both the antisymmetric (black, horizontal) and symmetric (gray, horizontal) states are shown.
The result is a transmission spectrum of NH3, sampled at the frequencies of the first QCL comb. To perform high-resolution spectroscopy, both QCL combs are scanned by increasing the laser currents using a “step-sweep” approach28, creating a set of 600 interleaved and normalized transmission spectra of NH3 which together yield a composite transmission spectrum with an ultimate resolution of 4.5 × 10−4 cm−1 (14 MHz) achievable in 7 min of total acquisition time.
The spectral region near \(\widetilde{\nu }\) ≈ 1060 cm−1 includes transitions from three different vibrational bands of NH3. Using a labeling scheme which indicates the number of quanta excited in each of the \({\nu }_{1}{\nu }_{2}{\nu }_{3}{\nu }_{4}\) normal vibrational modes of NH3, the transitions are described as (i) the fundamental \({\nu }_{2}\) ← \({\nu }_{0}\) band, corresponding to the 0100 ← 0000 transition, (ii) the \(2{\nu }_{2}\) ← \({\nu }_{2}\) hot band, corresponding to the 0200 ← 0100 transition and (iii) the \({\nu }_{2}\)+\({\nu }_{4}\) ← \({\nu }_{4}\) hot band, corresponding to the 0101 ← 0001 transition. Figure 1b shows a schematic of the vibrational levels probed here, with transition energies29 listed in wavenumbers. Shown are the energy values of both the symmetric (with respect to inversion, gray) and antisymmetric (black) states with respect to molecular inversion. In the case of the symmetric states, the \({\nu }_{4}\) and \(2{\nu }_{2}\) levels interchange their positions within the energy level diagram, as do the \({\nu }_{2}\) + \({\nu }_{4}\) and \(3{\nu }_{2}\) levels. Overall, the accessible vibrational states represent the first steps on a molecular vibrational ladder—or a series of energy levels which is useful for the study of overpopulation in non-thermal environments.
Here we generate the plasma in a research-grade, industrial reactor made of stainless steel by direct current (DC) discharge at a total power of 355 W ± 50 W. The discharge was located on a metal mesh of stainless steel in the top of the reactor. A workload made of stainless steel was also used to increase the discharge power and the stability of the plasma, and it was negatively biased relative to the reactor wall. A schematic of the plasma reactor is shown in Fig. 1a. Different mixtures of N2 and H2 gas precursors are delivered to the reactor at a constant mass flow rate of (500 ± 5) standard cubic centimeters per minute (sccm). The pressure inside the reactor is maintained at a constant value of 100 Pa ± 1 Pa using a back pressure controller and vacuum pump. The outer wall of the reactor is cooled to near room temperature at 295 K ± 1 K by a recirculating flow of water at 293.0 K ± 0.1 K, and the temperature of the inner wall (\({T}_{{{{{{\rm{wall}}}}}}}\)) is recorded by two temperature probes. Additionally, the temperature within the plasma at a blank stainless-steel working load (\({T}_{{{{{{\rm{load}}}}}}}\)) is also recorded using a third temperature probe.
Figure 2a shows the broadband transmission spectrum of NH3, measured for a plasma generated with precursor mass flow rates of 200 sccm of H2 and 300 sccm of N2. The strong absorption lines of the NH3 \({\nu }_{2}\,\) ← \({\nu }_{0}\) fundamental band are saturated at mixing ratios of H2/N2 ≈ 1 where the highest NH3 yield is observed. The fitted spectral model, calculated using the HITRAN2020 database30 and Voigt line shape functions, reveals absorption from the three different vibrational bands of NH3 illustrated in Fig. 1b. The fitted spectral model is the product of a two-zone transmission model: one spectrum for the non-thermal region between the inner walls of the reactor and another for the assumed thermal region beyond the inner walls. In the thermal region, we assume a single temperature for all degrees of freedom. We determine an NH3 number density in the non-thermal region, \(n\)n-th, by fitting a spectroscopic model that accounts for possible non-thermal distributions by floating the translational temperature, \({T}_{{{{{{\rm{trans}}}}}}}\), as well as the rotational temperature, \({T}_{{{{{{\rm{rot}}}}}}}\), and vibrational temperature, \({T}_{{{{{{\rm{vib}}}}}}}\), for the different vibrational bands. More details regarding the spectral model and fit are available in the Methods section. For the spectrum plotted in Fig. 2a, the resulting fit parameters and their estimated combined and relative uncertainties are listed in Table 1. The uncertainties reported in Table 1 highlight an advantage of the multi-line analysis31, performed here over approximately 125,000 unique spectral elements.
a Broadband transmission spectrum (Exp.; blue) of NH3 generated in an N2—H2 plasma (H2 mass flow: 200 sccm; N2 mass flow: 300 sccm; pressure: 100 Pa ± 1 Pa). Also shown are the Exp.-minus-Fit residuals (E − F; yellow), offset for clarity. The left inset shows a subsection of the E − F near strong NH3 absorption, and the middle inset shows another subsection near 1063 cm−1 with standard deviation of 0.0036. b–d Specific spectral regions showing transitions involving each 14NH3 vibrational band, shown along with the fitted spectral model (Fit; black) and residuals (E − F; yellow), again offset for clarity. b The \(2{\nu }_{2}\) ← \({\nu }_{2}\) hot band, and the \({\nu }_{2}\) ← \({\nu }_{0}\) fundamental band observed for 15NH3 at natural isotopic abundance. c The \({\nu }_{2}\) ← \({\nu }_{0}\) fundamental band. d The \({\nu }_{2}\) + \({\nu }_{4}\) ← \({\nu }_{4}\) hot band.
The broadband experiment-minus-fit residuals plotted in Fig. 2a (E − F; yellow line) show minor systematic residuals in the vicinity of the strong NH3 transitions with transmission near zero (Fig. 2a, left inset). Away from the saturated lines (Fig. 2a, middle inset), a representative standard deviation of the residuals yields a value of 0.0036, for a maximum signal-to-noise ratio (SNR) of 280:1. The interleaved step-sweep comb scanning approach provides redundant spectral information in the regions where a comb tooth frequency from the beginning of a step-sweep operation overlaps with the neighboring comb tooth frequency at the end of the step-sweep. We have experimented with fitting the data with and without the redundant spectral points and see no significant dependence of the fitted parameter values on this choice (i.e., parameters retrieved from fits with and without redundant spectral information agree with one another to within one standard deviation). Therefore, all fitted parameters (e.g., Table 1) are reported for spectral fits with some spectral overlap in adjacent comb teeth.
The transitions shown in Fig. 2b–d also serve to highlight an advantage of our broadband approach. As an example, if we assume an average global temperature for the non-thermal region of \({T}_{{{{{{\rm{trans}}}}}}}\) = \({T}_{{{{{{\rm{rot}}}}}}}\) = \({T}_{{{{{{\rm{vib}}}}}}}\) = 450 K, a line-by-line analysis of these respective transitions (as might be the case when narrow bandwidth CW lasers are used) would yield significantly different NH3 number densities, \(n\)n-th, when fitted for each respective spectral region. In such a scenario, single-transition fits of \(n\)n-th would differ between the spectral regions shown in Fig. 2b–d by a factor of six—potentially biasing conclusions on the efficiency of NH3 formation in non-thermal plasmas and impeding a quantitative comparison between different reactors.
NH3 number densities measured for different H2 mass flow fractions
In Fig. 3, we show the number density of NH3 in the non-thermal region (\(n\)n-th), obtained from our broadband spectral fits and plotted as a function of the H2 mass flow fraction. The H2 mass flow fraction is defined as \({\phi }_{{{{{{{\rm{H}}}}}}}_{2}}={\dot{m}}_{{{{{{{\rm{H}}}}}}}_{2}}/\left({\dot{m}}_{{{{{{{\rm{H}}}}}}}_{2}}+{\dot{m}}_{{{{{{{\rm{N}}}}}}}_{2}}\right)\), where \(\dot{m}\) is the mass flow of each precursor gas. The figure comprises data retrieved from the fitting of 27 unique spectra measured at different values of \({\phi }_{{{{{{{\rm{H}}}}}}}_{2}}\). The spectra were collected in two series, proceeding from either low to high values of \({\phi }_{{{{{{{\rm{H}}}}}}}_{2}}\) (left-to-right, blue circles) or high to low values of \({\phi }_{{{{{{{\rm{H}}}}}}}_{2}}\) (right-to-left, red triangles). The highest NH3 yield is observed on the H2-deficient side of the data set agrees with measurements performed on similar plasma reactors12. However, the maximum NH3 yield has also been observed in other types of reactors to be on the opposite, H2-abundant side6,7,10,11. The position of the maximum and the observed asymmetry with respect to \({\phi }_{{{{{{{\rm{H}}}}}}}_{2}}\) is influenced by the reactor wall material and process pressure, as well as the plasma electron energy and density7,32,33. The reported NH3 number densities span more than two orders of magnitude (see insets in Fig. 3), illustrating the wide dynamic range capabilities of QCL-DCS as a laser-based plasma diagnostic technique.
Mixed precursor gases were maintained inside the plasma reactor at a fixed pressure of 100 Pa ± 1 Pa. Measurements for the two data series (Data 1 and Data 2; red triangles and blue circles) proceeded in two distinct \({\phi }_{{{{{{{\rm{H}}}}}}}_{2}}\) directions (blue and red arrows). A modified Akima interpolation model60 (Interp.; black dashed line) is shown to illustrate the asymmetry in the observed data set, and zoomed-in traces near \({\phi }_{{{{{{{\rm{H}}}}}}}_{2}}\) = 0 and \({\phi }_{{{{{{{\rm{H}}}}}}}_{2}}\) = 1 are plotted as gray insets (linear y-axis). Error bars are combined standard uncertainty.
Non-thermal population of NH3 states
In Fig. 4, we show the fitted temperatures that partition population amongst the translational, rotational and vibrational states of NH3 found within the non-thermal region of the plasma reactor. Plotted in Fig. 4a are the fitted values of \({T}_{{{{{{\rm{vib}}}}}}}\) vs. \({\phi }_{{{{{{{\rm{H}}}}}}}_{2}}\). Generally, values of \({T}_{{{{{{\rm{vib}}}}}}}\) are between 400 K and 500 K, and we observe \({T}_{{{{{{\rm{vib}}}}}}}^{{\nu }_{4}}\) > \({T}_{{{{{{\rm{vib}}}}}}}^{{\nu }_{2}}\). Also plotted is a smoothing spline fitted to the average measured value of \({T}_{{{{{{\rm{wall}}}}}}}\). Figure 4b shows the fitted values of \({T}_{{{{{{\rm{rot}}}}}}}\) plotted vs. \({\phi }_{{{{{{{\rm{H}}}}}}}_{2}}\). The observed rotational temperatures reveal a general trend, where \({T}_{{{{{{\rm{rot}}}}}}}^{{\nu }_{4}}\) > \({T}_{{{{{{\rm{rot}}}}}}}^{{\nu }_{2}}\) > \({T}_{{{{{{\rm{rot}}}}}}}^{{\nu }_{0}}\). Two smoothing splines appear in Fig. 4b, each fitted to the average values of \({T}_{{{{{{\rm{load}}}}}}}\) and \({T}_{{{{{{\rm{wall}}}}}}}\), respectively. It should be noted that we have not observed any systematic difference in Doppler widths of hot band transitions compared to fundamental band transitions at a given plasma power and for the measurements SNR. This is expected because no external heating was applied to the plasma reactor, and the observed \({T}_{{{{{{\rm{load}}}}}}}\) > \({T}_{{{{{{\rm{wall}}}}}}}\) is mainly due to the negative bias applied to the working load. Furthermore, for the \(2{\nu }_{2}\) ← \({\nu }_{2}\) and \({\nu }_{2}\) + \({\nu }_{4}\) ← \({\nu }_{4}\) hot bands, values of \({T}_{{{{{{\rm{vib}}}}}}}\) and \({T}_{{{{{{\rm{rot}}}}}}}\) are only reported for spectra with an observed SNR greater than or equal to four for the strongest rovibrational transition within each respective hot band. The choice of SNR > 4 is rather arbitrary, but roughly corresponds with the minimum SNR required for the fitting of band temperatures to converge.
a Vibrational temperatures (\({T}_{{{{{{\rm{vib}}}}}}}\)) for the \({\nu }_{4}\) (yellow triangles) and \({\nu }_{2}\) (red squares) progressions, plotted vs. \({\phi }_{{{{{{{\rm{H}}}}}}}_{2}}\). b Rotational temperatures (\({T}_{{{{{{\rm{rot}}}}}}}\)) for the transitions beginning in \({\nu }_{4}\) (yellow triangles), \({\nu }_{2}\) (red squares) and \({\nu }_{0}\) (blue diamonds) states, plotted vs. \({\phi }_{{{{{{{\rm{H}}}}}}}_{2}}\). c Translational temperatures in the non-thermal (\({T}_{{{{{{\rm{trans}}}}}}}\), black circles) and thermal (\(T\)th, gray filled circles) regions, plotted vs. \({\phi }_{{{{{{{\rm{H}}}}}}}_{2}}\). Also plotted in a–c are the smoothed values of \({T}_{{{{{{\rm{load}}}}}}}\) (blue dashed line) and \({T}_{{{{{{\rm{wall}}}}}}}\) (magenta dashed lines). The smoothed values of \({T}_{{{{{{\rm{load}}}}}}}\) are only visible in b, where higher temperatures are shown.
Plotted in Fig. 4c are the fitted values for NH3 translational temperatures in the non-thermal (\({T}_{{{{{{\rm{trans}}}}}}}\)) and thermal (\({T}_{{{{{{\rm{th}}}}}}}\), in regions beyond the inner walls of the reactor) regions of the line-of-sight. In the thermal region, we assume a single temperature for all degrees of freedom (i.e., \({T}_{{{{{{\rm{th}}}}}}}\) = \({T}_{{{{{{\rm{rot}}}}}}}\) = \({T}_{{{{{{\rm{vib}}}}}}}\)), and the fitted results reveal a temperature for the thermal region that is consistent with the room temperature and the outer plasma-reactor walls, \({T}_{{{{{{\rm{th}}}}}}}\) ≈ 300 K. The non-thermal region, where NH3 is formed, shows a similar trend in \({T}_{{{{{{\rm{trans}}}}}}}\) vs. \({\phi }_{{{{{{{\rm{H}}}}}}}_{2}}\) to that observed for the NH3 number density, \(n\)n-th, in Fig. 3. Error bars are combined standard uncertainty for 1σ confidence level.
Discussion
The N2–H2 plasmas generated here are a case-study for plasma-activated N2 fixation to NH3. Therefore, it is instructive to postulate on the energy transfer dynamics that yield the non-thermal populations of rotational and vibrational states observed for NH3 products.
We partially attribute the measured non-thermal population of excited states—observed in the gas phase—to surface association reactions of radicals and atoms adsorbed on the reactor walls. These surface association reactions then release NH3 into the gas phase in vibrationally excited states. In addition to direct NH3 formation, surface association reactions can also form rovibrationally excited H2, as has already been reported in expanding plasmas34, and electron impact reactions can yield other vibrationally excited molecules, such as NH335.
Regardless of the formation mechanism, vibrationally excited NH3 in the gas phase is not likely to undergo rapid population redistribution through a vibrational–vibrational (V–V) energy transfer mechanism. Instead, and somewhat unique to NH3, experimental evidence suggests that vibrational–translational (V–T), vibrational–rotational (V–R), rotational–rotational (R–R) and rotational–translational (R–T) relaxation mechanisms are more likely36,37,38,39,40,41. This picture is consistent with the relatively low vibrational temperatures observed for NH3 in Fig. 4a, where we find \({T}_{{{{{{\rm{vib}}}}}}}\) < \({T}_{{{{{{\rm{rot}}}}}}}\) for both the \({\nu }_{4}\) and \({\nu }_{2}\) progressions. Indeed, NH3 behaves differently than other molecules like CO2 which exhibit rapid V-V relaxation processes along a vibrational ladder, leading to higher vibrational temperatures observed for CO2 formed in non-thermal plasmas42 than are observed here for NH3.
In the following discussion, we focus on two more observations: (i) The rotational temperature of the \({\nu }_{0}\) state is equal to—or in a few cases potentially lower than—the translational temperature, and (ii) the translational temperature appears to follow the same trend vs. \({\phi }_{{{{{{{\rm{H}}}}}}}_{2}}\) as the non-thermal NH3 number density.
Together, these observations point to the role of rapid V–T, V–R, R–R, and R–T energy transfer mechanisms in dictating the non-thermal population distribution of NH3 products. Initially, vibrationally excited NH3 is rapidly deactivated to translational and rotational degrees of freedom. Following V–V relaxation which takes place on longer time scales, any remaining vibrational energy in the lowest vibrational level of a given mode—here, \({\nu }_{2}\) or \({\nu }_{4}\)—is further dissipated into translational energy via V–T relaxation. If the translational degree of freedom is taken to be the main sink for excess vibrational energy contained in the initial NH3 products, this qualitatively explains observation (i), where \({T}_{{{{{{\rm{rot}}}}}}}^{{\nu }_{0}}\le {T}_{{{{{{\rm{trans}}}}}}}\).
Confidence in this proposed relaxation mechanism would be increased by reducing uncertainty in the \({T}_{{{{{\rm{rot}}}}}}^{{\nu }_{0}}\) and \({T}_{{{{{\rm{trans}}}}}}\) fitted parameters, for example by increasing spectral SNR. For most of the plasma conditions represented in Fig. 4, \({T}_{{{{{{\rm{rot}}}}}}}^{{\nu }_{0}}\) and \({T}_{{{{{{\rm{trans}}}}}}}\) are equivalent within their quoted 1σ uncertainties, and therefore the discussion could be strengthened by additional experiments or detailed modeling of the plasma chemistry to better quantify the relationship between \({T}_{{{{{{\rm{rot}}}}}}}^{{\nu }_{0}}\) and \({T}_{{{{{{\rm{trans}}}}}}}\).
Furthermore, NH3 is proposed as the major sink for excess vibrational energy found in all the plasma-activated molecules, due to relaxation via near-resonant V–V energy transfer between NH3 and a vibrationally excited N2 or H2 collider. Once the excess energy is transferred to NH3, the above-mentioned mechanisms of V–T, V–R, R–R, and R–T relaxation dominate. In such a scenario, energy is most efficiently dissipated into translation motion through NH3 relaxation channels. Ammonia is a polar molecule with a steep intermolecular potential energy curve for collisional processes, leading to efficient relaxation of internal energy into heat36,37. This suggests that differences in buffer gas composition would affect efficiency, and hence chemical reactivity within N2–H2 plasmas. Indeed, we anticipate that vibrationally excited N2 (\(\widetilde{\nu }\) = 2311 cm−1) and H2 (\(\widetilde{\nu }\) = 4160 cm−1) are both formed in the plasma, and could transfer their energy via rapid V-V mechanisms into NH3 rather than dissipating it by self-collision and internal V–T processes. The number of collisions required to achieve self-deactivation via V–T for NH3, H2 and N2, respectively, is approximately 5, 107 and 109 at a temperature of 300 K38. For comparison, the deactivation of NH3 by collision with N2 is measured to require only 670 collisions37. From these values, we would expect that deactivation of NH3 by collision with H2 would take greater than 670 collisions, as could also be inferred from looking at the respective vibrational frequencies of H2 and N2 (which differ by almost a factor of two). Therefore, the most efficient route to deactivation of vibrationally excited molecules in the plasma appears to be through collisions with NH3. This hypothesis is consistent with the following observation (ii), that \({T}_{{{{{{\rm{trans}}}}}}}\) appears to follow an asymmetry trend vs. \({\phi }_{{{{{{{\rm{H}}}}}}}_{2}}\) that is qualitatively like that of NH3 number density. As the energy sink molecule in our N2–H2 plasmas, greater number densities of NH3 result in a higher \({T}_{{{{{{\rm{trans}}}}}}}\).
The observation of similar trends in \({T}_{{{{{{\rm{trans}}}}}}}\) and \(n\)n-th when plotted vs. \({\phi }_{{{{{{{\rm{H}}}}}}}_{2}}\) provides additional support for the argument that V–T processes are largely responsible for depleting the anticipated initial population of excited vibrational states that occurs immediately following NH3 formation at the reactor surfaces. Contrast this trend with the impact that collisions between plasma-generated vibrationally excited NH3 and electrons would have on the observed steady-state populations: If processes such as scattering, momentum transfer, dissociation, and excitation of rotational and vibrational degrees of freedom were induced by electron-NH3 collisions, we would expect a monotonic change in \({T}_{{{{{{\rm{trans}}}}}}}\) vs. \({\phi }_{{{{{{{\rm{H}}}}}}}_{2}}\)35. Additionally, the rate coefficient for the dissociation of NH3 by electrons—calculated at an electron temperature of 0.31 eV observed for similar plasma reactors43—is roughly three orders of magnitude lower than the predicted V-T relaxation rate32. Thus, V–T is likely the dominant relaxation process for NH3 once generated in N2–H2 plasma.
Outlook
We demonstrate that quantum cascade laser dual-comb spectroscopy (QCL-DCS) can provide precision measurements of number densities and non-thermal population distributions across the translational, rotational and vibrational degrees of freedom of molecules confined to a reactive plasma environment. This is achieved here for a broad optical bandwidth by fast spectra interleaving, resulting in high resolution spectroscopy. With other chip-based laser sources like the long-wave infrared QCLs used here, DCS could also be employed as a plasma diagnostic in the mid-wave infrared using interband cascade lasers44 or in the THz regime using QCLs45. When combined with injection-locked electro-optic comb generators in the short-wave infrared46, it may become possible to use DCS as a plasma diagnostic anywhere across the wavelength range of 1–100 μm, choosing between a series of distinct yet compact instruments. This would advance the field of laser-based plasma diagnostics beyond the current state-of-the-art—where only a few pre-selected rovibrational transitions of a single molecular species are observed—by enabling observations of several vibrational bands of multiple species, without sacrificing spectral resolution or measurement speed.
In this demonstration, we begin to unravel the complex energy transfer mechanisms that result from a non-thermal population of energy levels in plasma-activated ammonia. Specifically, we highlight the role of various energy transfer mechanisms in partitioning population densities amongst quantized states, a process which significantly affects chemical reactivity. This opens the door to further systematic studies of plasma-activated processes involving NH3 generation, water-enhanced NH3 synthesis47, or the conversion of carbon dioxide to high value-added chemical products like renewable fuels. Furthermore, such a quantum-state-resolved picture of molecules within reactive environments will be of high importance for understanding other plasma driven chemical syntheses and transformations. When combined with plasma models and theory, such quantum-state-resolved experiments will address clear knowledge gaps in plasma driven NH3 formations48.
Methods
Quantum cascade laser dual-comb spectroscopy setup
The dual-comb source (IRsweep IRis-core) emitted in the spectral range from 1035 cm−1 to 1085 cm−1 with repetition rates of frep ≈ 7.417 GHz, a difference in repetition rates of Δfrep = 2.1 MHz and average output optical powers ≥100 mW. The QCL outputs were attenuated by approximately tenfold using neutral density filters and aligned to create two dual-comb paths: reference and probe. Polarizers were used in each recombined QCL beam path to match interferogram intensities, and the dual-comb beams were focused onto photodetectors using off-axis parabolic mirrors with focal lengths of 25.4 mm.
Transmission spectra, T(\(\widetilde{\nu }\)) were calculated by squaring the ratio \({I}^{b}\left(\widetilde{\nu }\right)/{I}_{0}^{b}\left(\widetilde{\nu }\right)\), where \({I}^{b}\left(\widetilde{\nu }\right)\) is the intensity of the multi-heterodyne beat notes measured after passing through a plasma containing both N2 and H2 (the sample spectrum) and \({I}_{0}^{b}\left(\widetilde{\nu }\right)\) is the intensity measured in a pure N2 or H2 plasma (the background spectrum) where no NH3 is formed. The squaring of the ratio is required when only one of the two interfering combs in the sample channel passes through the sample, thus creating a phase-sensitive configuration49. To suppress laser intensity noise and frequency noise, all measured intensities are normalized by the simultaneously measured intensities in the reference channel where both combs bypass the reactor.
High-resolution spectra were obtained by spectral interleaving of 600 measurements following the “step-sweep” approach28, yielding a spectral point spacing of 4.6 × 10−4 cm−1 (14 MHz) in a total measurement time of seven minutes. The absolute frequency axis was calibrated by matching the offset frequency, foff and frep of the first measurement step to a pair of NH3 line positions retrieved from the HITRAN2020 database30. To correct for a residual drift of the measured wavenumber axis with interleaving step number, the measured changes in foff in every step were corrected by a constant factor of 0.9942 and 0.9972, respectively, for measurements taken with two slightly different stabilization times on different days. These factors were determined as the linear slope in the difference between measured and reference absorption peak positions of NH3 from the HITRAN2020 database30 plotted against the step number at which the absorption peak was measured. Hence, three fitting parameters (frep, foff, and Δfoff) were used to assign approximately 125 000 spectral datapoints. After such calibration, the difference between found peak positions and those listed in the HITRAN2020 database30 were <4.3 × 10−4 cm−1 (or 13 MHz).
The interleaved spectra of plasma samples showed a constant offset (>5% transmission) in amplitude and linear slope in phase. These background signals varied on timescales from minutes to hours, as well as on a timescale of seconds, or between interleaving steps. The slower variations are likely due to thermal drifts. Since the spectrometer and the reactor are not mechanically connected, thermal expansion in the plasma reactor can change detector alignment and therefore signal levels. The origin of the fast drifts could not be identified. The constant offset in amplitude and linear slope in phase were both removed in post-processing from every interleaving step. To this end, absorption features were first masked out. Then, the median value of transmission of the remaining spectral datapoints was subtracted, as well as a linear function fitted to the phase. This procedure reduced the root-mean-square noise on the measured transmission to 0.0045, with the remaining noise dominated by optical fringing in the multi-pass cell, which was further fitted by a series of polynomial baseline functions.
Broadband spectral model and analysis
In absorption spectroscopy, the laser intensity spectrum, \(I\left(\widetilde{\nu }\right)\), after having propagated along an absorption pathlength, \(L\), and being normalized by a background intensity spectrum, \({I}_{0}\left(\widetilde{\nu }\right)\), follows an exponential decay described by the Beer–Lambert law. Here, the ratio \(I\left(\widetilde{\nu }\right)/{I}_{0}\left(\widetilde{\nu }\right)\) is taken to be the experimental observable for asynchronous DCS, \({\left\{{I}^{b}\left(\widetilde{\nu }\right)/{I}_{0}^{b}\left(\widetilde{\nu }\right)\right\}}^{2}\). The Beer–Lambert law is stated in Eq. (1), and includes a total number density of absorbers, \(n\), a spectral line intensity, \({S}_{{ij}}\), for a transition connecting a lower state, \(i\), with an upper state, \(j\), and an area-normalized line shape function, \(g\left(\widetilde{\nu }\right)\):
Above, \(\widetilde{\nu }\) is frequency in wavenumbers. The transition intensity, \({S}_{{ij}}\), is related to the difference in number density between the lower and upper states50 by the following equation:
In Eq. (2), \({I}_{{{{{{\rm{a}}}}}}}\) is the isotopic abundance of the species involved in the specific transition, \({n}_{i}\) is the lower-state number density, \({n}_{j}\) is the upper-state number density, \({B}_{{ij}}\) and \({B}_{{ji}}\) are the Einstein \(B\)-coefficients for induced absorption and emission, respectively, \(h\) is the Plank constant, \({\widetilde{\nu }}_{{ij}}\) is the transition frequency, and \(c\) is the speed of light. Note that \({g}_{i}{B}_{{ij}}={g}_{j}{B}_{{ji}}\) and \({A}_{{ij}}=8\pi h{\widetilde{\nu }}_{{ij}}^{3}{B}_{{ji}}\), where \({g}_{i}\) is the lower-state statistical weight, \({g}_{j}\) is the upper-state statistical weight, and \({A}_{{ij}}\) is the Einstein \(A\)-coefficient for spontaneous emission.
Following the procedure of Klarenaar et al. 42, we write the number density \(n\) for each energy level \(l\), \({n}_{l}\), where \(l\) = \(i\) or \(l\) = \(j\), as:
Above, \(J\) is the rotational quantum number and \(m\) is the index of vibrational modes (\({v}_{m}\) = 1, 2, 3, or 4). The fraction of molecules, \({n}_{l}/n\), in both rotational state \(J\), \({\phi }_{{{{{{\rm{rot}}}}}},J}\), and vibrational mode \({v}_{m}\), \({\phi }_{{{{{{\rm{vib}}}}}},{v}_{m}}\), is normalized by the total internal partition sum, \({Q}_{{{{{{\rm{tot}}}}}}}={Q}_{{{{{{\rm{rot}}}}}}}{Q}_{{{{{{\rm{vib}}}}}}}\). Here, the internal partition sums for rotation and vibration are50,51,52:
In Eqs. (4)–(5), \({T}_{{{{{{\rm{rot}}}}}}}\) is the rotational temperature, \({T}_{{{{{{\rm{vib}}}}}}}\) is the vibrational temperature, \({g}_{{{{{{\rm{s}}}}}}}\) and \({g}_{{{{{{\rm{in}}}}}}}\) are the state-dependent and state-independent weights, \({g}_{{v}_{m}}\) is the degeneracy for the fundamental vibration \({v}_{m}\), and \({G}_{{v}_{m}}\) is the term symbol for vibration \({v}_{m}\). To calculate \({Q}_{{{{{{\rm{rot}}}}}}}\) for ammonia (NH3), we use values for \({g}_{{{{{{\rm{s}}}}}}}\) and \({g}_{{{{{{\rm{in}}}}}}}\) from Šimečková et al.50 and sum over all rotational states listed in ExoMol53,54, up to Jmax = 43. To calculate \({Q}_{{{{{{\rm{vib}}}}}}}\) for NH3, we use values for \({G}_{{v}_{m}}\) from Polyansky et al.55 and degeneracy factors \({g}_{{v}_{m}}\) derived from the D3h point group56. At values of \(T\) < 1000 K, when letting \(T={T}_{{{{{{\rm{rot}}}}}}}={T}_{{{{{{\rm{vib}}}}}}}\), our calculation of \({S}_{{ij}}\) using Eq. (2) has a relative deviation from the temperature-dependent values calculated from HITRAN2020 parameters30 of <6%. This bias was corrected for each vibrational band and is ascribed to the use of an incomplete list of energy levels in our total partition function summations.
For the line shape function, \(g\left(\widetilde{\nu }\right)\), we use a Voigt function with a Doppler-broadened half-width at half-maximum of:
where \({N}_{A}\) is the Avogadro constant, \({T}_{{{{{{\rm{trans}}}}}}}\) is the translational temperature, and \(M\) is the molecular molar mass. Here we assume a single, shared value for \({T}_{{{{{{\rm{trans}}}}}}}\) across all transitions. The Lorentzian term for the Voigt function was calculated using the measured gas pressure and the temperature dependent air-broadening coefficients for NH3 from HITRAN202030.
For the non-thermal region of the plasma reactor, we use the above equations to model and fit the absorption by 86 total transitions belonging to the \({\nu }_{2}\) ← \({\nu }_{0}\) fundamental band (20 transitions), the \(2{\nu }_{2}\) ← \({\nu }_{2}\) hot band (15 transitions), and the \({\nu }_{2}\) + \({\nu }_{4}\) ← \({\nu }_{4}\) hot band (51 transitions). All other transitions meeting an intensity threshold criterion of \(\left(5\times {10}^{-5}\right)\times {S}_{{ij},\max }\left({T}_{{{{{{\rm{rot}}}}}}},{T}_{{vib}}\right)\), where \({S}_{{ij},\max }\) is the maximum temperature-dependent transition intensity generated from within the list of the 86 targeted lines, are simulated assuming \(T={T}_{{{{{{\rm{trans}}}}}}}={T}_{{{{{{\rm{rot}}}}}}}={T}_{{{{{{\rm{vib}}}}}}}\) at a temperature equal to the fitted value of \({T}_{{{{{{\rm{trans}}}}}}}\).
Based on machine drawings of the physical dimensions of the plasma reactor, we estimate the single-pass path length of the non-thermal region along the line-of-sight to be 64.0 cm—equivalent to the physical distance between the hot inner walls of the reactor. For a single-pass total optical path length of 79.0 cm ± 0.5 cm, we estimate a thermal region beyond the hot inner walls to be 15.0 cm in length, resulting in a fractional thermal path length of \({f}_{{{{{{\rm{th}}}}}}}\) = 0.190. Again, following the procedure of Klarenaar et al.42, we fit a single thermal temperature, \({T}_{{{{{{\rm{th}}}}}}}\), for the thermal region with a lower bound equal to the observed room temperature of 295 K and an upper bound equal to the translational temperature of the non-thermal region, \({T}_{{{{{{\rm{trans}}}}}}}\). The combined model for the observed transmission signal is then the product of the respective transmission models for the thermal and non-thermal regions, both using the same set of rovibrational transitions. Thermal and non-thermal number densities are calculated assuming the ideal gas law, using either \({T}_{{{{{{\rm{th}}}}}}}\) or \({T}_{{{{{{\rm{trans}}}}}}}\).
Uncertainty analysis
We adopt a probabilistic approach to the uncertainty propagation57, using Monte Carlo simulation methods and fitting to produce a distribution of output values that are the result from models generated by randomly drawn inputs. Components of the spectral reference data used to model NH3 absorption are evaluated at randomly selected values assuming a normal distribution with a standard deviation equal to the upper-limit HITRAN2020 uncertainty codes30. For example, the transition intensity error codes for most of the lines included in our model have a relative uncertainty of <20%. For the physical input parameters optical path length and pressure, we also randomly draw values from normal distributions. A summary of model input parameters with sizeable standard deviations, listed as relative uncertainties, is shown in Table 2.
During fitting, we use the HITRAN2020 isotopic abundance value to model all 15NH3 lines. In determining \({T}_{{{{{{\rm{trans}}}}}}}\) from the Doppler-broadened line widths, uncertainties in \({\widetilde{\nu }}_{{ij}}\) and \(M\) are considered negligible. Also, at the sample pressure of 100 Pa ± 1 Pa, uncertainties in the collisional-broadening parameters are considered negligible. Finally, the uncertainties in the energies of the individual energy levels are considered negligible. At the observed experimental signal-to-noise ratio, we find no systematic deviations in the residuals that would indicate the need to simulate line profiles beyond the Voigt profile, and we maintain a fixed value for the fractional thermal path length, \({f}_{{{{{{\rm{th}}}}}}}\), when drawing random values for the total path length, \(L\).
For the spectra where lines from all three of the vibrational bands have a high signal-to-noise ratio, we model and fit 100 unique Monte Carlo model simulations drawn from the uncertain input parameters. The reported values and uncertainties for each floated parameter are taken to be the mean values and standard deviations resulting from the 100 fits. The Monte Carlo routine also included variations in the initial values for each floated parameter. For spectra where only one or two vibrational bands are of sufficiently high SNR to fit, the number of Monte Carlo simulations and fits is reduced to 10.
When the signal-to-noise ratio (SNR) becomes small for a specific vibrational band (i.e., when SNR < 4 for the strongest transitions), we fix the values for \({T}_{{{{{{\rm{rot}}}}}}}\) and \({T}_{{{{{{\rm{vib}}}}}}}\) for that band which are randomly drawn from the weighted mean and weighted standard deviation of the fitted values from the other spectra with SNR ≥ 4.
Tables of initial parameters and drawing distributions used for each fitted spectrum, along with the mean values and standard deviations that resulted from the Monte Carlo simulation and fitting routine, are provided as Supplementary Data 1.
Preliminary line-by-line spectral analysis
Prior to the broadband spectral modeling and fitting described above and reported in the Results section, a preliminary line-by-line analysis was useful in estimating initial guesses for parameters like \({T}_{{{{{{\rm{trans}}}}}}}\) and \({T}_{{{{{{\rm{rot}}}}}}}\), and for identifying transitions—particularly hot-band transitions—that required manual adjustments to their HITRAN202030 frequencies to accommodate our assigned wavenumber axis. A list of transition frequency adjustments applied to our broadband model is provided as Supplementary Data 2.
Here we focus on a Boltzmann plot analysis to determine initial values of \({T}_{{{{{{\rm{rot}}}}}}}\). By plotting the natural logarithm of the lower-state number density, \({n}_{i}\), normalized by the lower-state statistical weight, \({g}_{i}\) (i.e., the quantity \({{{{\mathrm{ln}}}}}\left({n}_{i}/{g}_{i}\right)\)), versus the lower-state energy, \({E}_{i}\), rotational temperatures could be estimated from the band-specific Boltzmann plot slopes. The Boltzmann plot analysis is presented here in Fig. 5.
Rotational temperatures, \({T}_{{{{{{\rm{rot}}}}}}}\), were estimated for three rovibrational bands of NH3, including the ν2 ← ν0 fundamental band (black dots), the 2ν2 ← ν2 hot band (black squares), and the ν4+ν2 ← ν4 hot band (black diamonds). Linear fits are also shown (red lines). Two data sets for each vibrational band are presented, each of which was collected at \({\phi }_{{{{{{{\rm{H}}}}}}}_{2}}\) = 0.6 and recorded on successive days. Error bars represent 1σ fit precision in the area of the line profile (type-A evaluation only).
To create Fig. 5, individual lines were fit with a Voigt line shape function, with the fit parameters including the Doppler-broadened half-width at half-maximum and the line center. The translational temperature (Ttrans) was evaluated from the Doppler width using Eq. (6), while \({n}_{i}\) was determined from the integral of the absorption profile and the HITRAN2020 parameters \({A}_{{ij}}\) and \({\widetilde{\nu }}_{{ij}}\).
More detailed Boltzmann plot expressions are introduced below. The statistical-weight-normalized number density in state \(i\) is described by the Boltzmann law:
Remembering the expressions \({g}_{i}{B}_{{ij}}={g}_{j}{B}_{{ji}}\) and \({A}_{{ij}}=8\pi h{\widetilde{\nu }}_{{ij}}^{3}{B}_{{ji}}\) noted earlier, and by rearrangement of the Beer–Lambert law expressed in Eq. (1) and the transition intensity defined in Eq. (2)50, we can also write the ratio \({n}_{i}/{g}_{i}\) in terms of the experimental observables \({I}_{0}\left(\widetilde{\nu }\right)\) and \(I\left(\widetilde{\nu }\right)\):
In Eq. (8), we assume that \(\left({n}_{i}\frac{{g}_{j}}{{g}_{i}}-{n}_{j}\right)\approx {n}_{i}\frac{{g}_{j}}{{g}_{i}}\) if \({n}_{i} \, \gg \, {n}_{j}\). This assumption is reasonable for the transitions studied here when the sample temperature is near 300 K. At higher temperatures, however, this is not a safe assumption, and therefore the rotational temperatures estimated from the Boltzmann plot in Fig. 5 are used as initial values in the broadband spectral model and fit. Taking the natural logarithm of both sides of Eq. (7)—and using Eq. (8) along with reference quantities from HITRAN202030 to calculate \({n}_{i}/{g}_{i}\) from the fits of our experimental data—yields the desired Eq. (9), where the slopes of the data plotted in Fig. 5 are inversely proportional to the estimated rotational temperatures for each vibrational band:
Note that the initial Boltzmann plot analysis does not account for the thermal population outside the reactor core.
References
IEA. Ammonia Technology Roadmap, IEA, Paris https://www.iea.org/reports/ammonia-technology-roadmap, License: CC BY 4.0. (2021).
Erisman, J. W., Sutton, M. A., Galloway, J., Klimont, Z. & Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 1, 636–639 (2008).
Hong, J., Prawer, S. & Murphy, A. B. Plasma catalysis as an alternative route for ammonia production: status, mechanisms, and prospects for progress. ACS Sustain. Chem. Eng. 6, 15–31 (2018).
Bogaerts, A. & Neyts, E. C. Plasma technology: an emerging technology for energy storage. ACS Energy Lett. 3, 1013–1027 (2018).
Touchard, S., Mougenot, J., Rond, C., Hassouni, K. & Bonnin, X. AMMONX: a kinetic ammonia production scheme for EIRENE implementation. Nucl. Mater. Energy 18, 12–17 (2019).
Gordiets, B., Ferreira, C. M., Pinheiro, M. J. & Ricard, A. Self-consistent kinetic model of low-pressure—flowing discharges: II. surface processes and densities of N, H, species. Plasma Sources Sci. Technol. 7, 379–388 (1998).
van Helden, J. H. et al. Detailed study of the plasma-activated catalytic generation of ammonia in N2–H2 plasmas. J. Appl. Phys. 101, 043305 (2007).
Devasia, D., Das, A., Mohan, V. & Jain, P. K. Control of chemical reaction pathways by light-matter coupling. Annu. Rev. Phys. Chem. 72, 423–443 (2021).
Bogaerts, A. et al. The 2020 plasma catalysis roadmap. J. Phys. D Appl. Phys. 53, 443001 (2020).
Vankan, P., Rutten, T., Mazouffre, S., Schram, D. C. & Engeln, R. Absolute density measurements of ammonia produced via plasma-activated catalysis. Appl. Phys. Lett. 81, 418–420 (2002).
Puth, A. et al. Spectroscopic investigations of plasma nitrocarburizing processes using an active screen made of carbon in a model reactor. Plasma Sources Sci. Technol. 27, 075017 (2018).
Dalke, A. et al. Solid carbon active screen plasma nitrocarburizing of AISI 316L stainless steel: influence of N2–H2 gas composition on structure and properties of expanded austenite. Surf. Coat. Technol. 357, 1060–1068 (2019).
Hempel, F., Davies, P. B., Loffhagen, D., Mechold, L. & Röpcke, J. Diagnostic studies of H2–Ar–N2 microwave plasmas containing methane or methanol using tunable infrared diode laser absorption spectroscopy. Plasma Sources Sci. Technol. 12, S98–S110 (2003).
Phillips, M. C., Myers, T. L., Johnson, T. J. & Weise, D. R. In-situ measurements of pyrolysis and combustion gases from biomass burning using swept wavelength external cavity quantum cascade lasers. Opt. Express 28, 8680–8700 (2020).
Witsch, D. et al. The rotationally resolved infrared spectrum of TiO and its isotopologues. J. Mol. Spectrosc. 377, 111439 (2021).
Golkowski, M. et al. Hydrogen-Peroxide-Enhanced Nonthermal Plasma Effluent for Biomedical Applications. IEEE Trans. Plasma Sci. 40, 1984–1991 (2012).
Abbas, M. A. et al. Time-resolved mid-infrared dual-comb spectroscopy. Sci. Rep. 9, 17247 (2019).
Bergevin, J. et al. Dual-comb spectroscopy of laser-induced plasmas. Nat. Commun. 9, 1273 (2018).
Capitelli, M., Capitelli, F. & Eletskii, A. Non-equilibrium and equilibrium problems in laser-induced plasmas. Spectrochim. Acta Part B 55, 559–574 (2000).
Fiévet, R., Voelkel, S., Koo, H., Raman, V. & Varghese, R. L. Effect of thermal nonequilibrium on ignition in scramjet combustors. Proc. Combust. Inst. 36, 2901–2910 (2017).
Wright, S. O. M., Waldmann, I. & Yurchenko, S. N. Non-local thermal equilibrium spectra of atmospheric molecules for exoplanets. MNRAS 512, 2911–2924 (2022).
Hanson, R. K. Applications of quantitative laser sensors to kinetics, propulsion and practical energy systems. Proc. Combust. Inst. 33, 1–4 (2011).
Villares, G., Hugi, A., Blaser, S. & Faist, J. Dual-comb spectroscopy based on quantum-cascade-laser frequency combs. Nat. Commun. 5, 5192 (2014).
Hayden, J. et al. Mid-infrared dual-comb spectroscopy with quantum cascade lasers. APL Photonics 9, 031101 (2024).
Agner, J. A. et al. High-resolution spectroscopic measurements of cold samples in supersonic beams using a QCL dual-comb spectrometer. Mol. Phys. 120, e2094297 (2022).
Komagata, K. N., Wittwer, V. J., Südmeyer, T., Emmenegger, L. & Gianella, M. Absolute frequency referencing for swept dual-comb spectroscopy with midinfrared quantum cascade lasers. Phys. Rev. Res. 5, 013047 (2023).
Klocke, J. L. et al. Single-shot sub-microsecond mid-infrared spectroscopy on protein reactions with quantum cascade laser frequency combs. Anal. Chem. 90, 10494–10500 (2018).
Lepère, M. et al. A mid-infrared dual-comb spectrometer in step-sweep mode for high-resolution molecular spectroscopy. J. Quant. Spectrosc. Radiat. Transf. 287, 108239 (2022).
Yurchenko, S. N., Barber, R. J. & Tennyson, J. A variationally computed line list for hot NH3. Mon. Not. R. Astron. Soc. 413, 1828–1834 (2011).
Gordon, I. E. et al. The HITRAN2020 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 277, 107949 (2022).
Adler, F. et al. Mid-infrared Fourier transform spectroscopy with a broadband frequency comb. Opt. Express 18, 21861–21873 (2010).
Hong, J. et al. Corrigendum: kinetic modelling of NH3 production in N2–H2 non-equilibrium atmospheric-pressure plasma catalysis. J. Phys. D Appl. Phys. 51, 109501 (2018). (2017 J. Phys. D: Appl. Phys. 50, 154005).
Ben Yaala, M. et al. Plasma-activated catalytic formation of ammonia from N2–H2: influence of temperature and noble gas addition. Nucl. Fusion 60, 016026 (2020).
Vankan, P., Schram, D. C. & Engeln, R. Relaxation behavior of rovibrationally excited H2 in a rarefied expansion. J. Chem. Phys. 121, 9876–9884 (2004).
Itikawa, Y. Cross sections for electron collisions with ammonia. J. Phys. Chem. Ref. Data 46, 043103 (2017).
Rowlinson, J. S. The second virial coefficients of polar gases. Trans. Faraday Soc. 45, 974–984 (1949).
Hovis, F. E. & Moore, C. B. Vibrational relaxation of NH3(v2). J. Chem. Phys. 69, 4947–4950 (1978).
Lambert, J. D. Vibration-translation and vibration-rotation energy transfer in polyatomic molecules. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 68, 364–373 (1972).
Dubé, P. & Reid, J. Vibrational relaxation of the 2ν2 level of NH3. J. Chem. Phys. 90, 2892–2899 (1989).
Shultz, M. J. & Wei, J. Infrared, resonance enhanced multiphoton ionization double resonance detection of energy transfer in NH3. J. Chem. Phys. 92, 5951–5958 (1990).
Abel, B., Coy, S. L., Klaassen, J. J. & Steinfeild, J. I. State-to-state rotational energy-transfer measurements in the v2 = 1 state of ammonia by infrared-infrared double resonance. J. Chem. Phys. 96, 8236–8250 (1992).
Klarenaar, B. L. M. et al. Time evolution of vibrational temperatures in a CO2 glow discharge measured with infrared absorption spectroscopy. Plasma Sources Sci. Technol. 26, 115008 (2017).
Hannemann, M. et al. Langmuir probe and optical diagnostics of active screen N2–H2 plasma nitriding processes with admixture of CH4. Surf. Coat. Technol. 235, 561–569 (2013).
Sterczewski, L. A. et al. Mid-infrared dual-comb spectroscopy with interband cascade lasers. Opt. Lett. 44, 2113–2116 (2019).
Sterczewski, L. A. et al. Terahertz spectroscopy of gas mixtures with dual quantum cascade laser frequency combs. ACS Photonics 7, 1082–1087 (2020).
Van Gasse, K., et al. An on-chip III-V-semiconductor-on-silicon laser frequency comb for gas-phase molecular spectroscopy in real-time. Preprint at arXiv https://doi.org/10.48550/arXiv.2006.15113 (2020).
Vervloessem, E. et al. NH3 and HNOx formation and loss in nitrogen fixation from air with water vapor by nonequilibrium plasma. ACS Sustain. Chem. Eng. 11, 4289–4298 (2023).
Whitehead, J. C. Plasma–catalysis: the known knowns, the known unknowns and the unknown unknowns. J. Phys. D Appl. Phys. 49, 243001 (2016).
Coddington, I., Newbury, N. & Swann, W. Dual-comb spectroscopy. Optica 3, 414–426 (2016).
Šimečková, M., Jacquemart, D., Rothman, L. S., Gamache, R. R. & Goldman, A. Einstein A-coefficients and statistical weights for molecular absorption transitions in the HITRAN database. J. Quant. Spectrosc. Radiat. Transf. 98, 130–155 (2006).
Herzberg, G. Infrared and Raman Spectra of Polyatomic Molecules (Lancaster Press, Lancaster, PA, 1945).
Gamache, R. R. et al. Total internal partition sums for 166 isotopologues of 51 molecules important in planetary atmospheres: application to HITRAN2016 and beyond. J. Quant. Spectrosc. Radiat. Transf. 203, 70–87 (2017).
Al Derzi, A. R., Furtenbacher, T., Tennyson, J., Yurchenko, S. N. & Császár, A. G. MARVEL analysis of the measured high-resolution spectra of 14NH3. J. Quant. Spectrosc. Radiat. Transf. 161, 117–130 (2015).
Coles, P. A., Yurchenko, S. N. & Tennyson, J. ExoMol molecular line lists—XXXV. A rotation-vibration line list for hot ammonia. Mon. Not. R. Astron. Soc. 490, 4638–4647 (2019).
Polyansky, O. L. et al. Calculation of rotation-vibration energy levels of the ammonia molecule based on an ab initio potential energy surface. J. Mol. Spectrosc. 327, 21–30 (2016).
Philip, R. & Bunker, P. J. Molecular Symmetry and Spectroscopy (NRC Research Press, Ottawa, 2006).
Possolo, A. & Iyer, H. K. Invited article: concepts and tools for the evolution of measurment uncertianty. Rev. Sci. Instrum. 88, 011301 (2017).
Aroui, H., Nouri, S. & Bouanich, J.-P. NH3 self-broadening coefficients in the ν2 and ν4 bands and line intensities in the ν2 band. J. Mol. Spectrosc. 220, 248–258 (2003).
Down, M. J. et al. Re-analysis of ammonia spectra updating the HITRAN 14NH3 database. J. Quant. Spectrosc. Radiat. Transf. 130, 260–272 (2013).
Akima, H. A new method of interpolation and smooth curve fitting based on local procedures. J. Assoc. Comput. Mach. 17, 589–602 (1970).
Acknowledgements
The INP acknowledges funding from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—project No SA 4483/1–1. IRsweep acknowledges funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 101032761. AJF acknowledges both NIST and the INP for supporting a research visit to INP. We thank J.T. Hodges, E.A. Adkins, B.R. Washburn, M. Becker, and A. Foltynowicz for commenting on the paper.
Author information
Authors and Affiliations
Contributions
According to CRediT (Contributor Roles Taxonomy), I. Sadiek. has contributed to Formal analysis; Investigation; Visualization; Data curation; Conceptualization; and Writing—original draft, A. J. Fleisher has contributed to Formal analysis; Investigation; Validation; Visualization; and Writing—original draft; J. Hayden has contributed to Investigation; Data curation; Software; Methodology; and Writing—review & editing, X. Huang has contributed to Methodology, A. Hugi has contributed to Resources, R. Engeln has contributed to Writing—review & editing, N. Lang has contributed to Project administration; and Writing—review & editing, J. H. van Helden has contributed to Resources; Writing—review & editing; Conceptualization; and Supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sadiek, I., Fleisher, A.J., Hayden, J. et al. Dual-comb spectroscopy of ammonia formation in non-thermal plasmas. Commun Chem 7, 110 (2024). https://doi.org/10.1038/s42004-024-01190-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-024-01190-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.