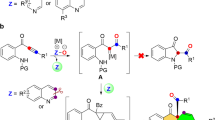
Abstract
One-carbon homologation reactions based on one-carbon insertion into the N−O bond of heterocycles have received tremendous interest over the past decades. However, these protocols have to rely on the use of hazardous and not easily accessible diazo compounds as precursors, and examples of the relevant asymmetric catalysis have not been reported. Here we show that a copper-catalyzed intermolecular formal (5 + 1) annulation of 1,5-diynes with 1,2,5-oxadiazoles involving one-carbon insertion into the heterocyclic N−O bond via non-diazo approach. This method enables practical and atom-economic synthesis of valuable pyrrole-substituted oxadiazines in generally moderate to good yields under mild reaction conditions. In addition, the possibility of such an asymmetric formal (5 + 1) annulation also emerges.
Similar content being viewed by others
Introduction
Oxadiazines are a class of important heterocyclic compounds, which are widely present in drug molecules and show good activities in anticancer, antiviral, antibacterial and weed control1,2,3,4,5,6,7,8,9,10,11,12,13,14. As a result, the development of efficient methods for synthesis of oxadiazines continues to draw a great deal of interest from the synthetic community. Although a range of methods have been developed for the construction of oxadiazines15,16,17,18,19,20,21,22,23,24, only a few reports involved the synthesis of 1,2,5-oxadiazines, which are still challenging to be accessed due to the lack of efficient method.
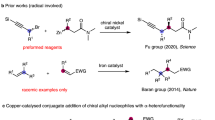
One-carbon homologation reactions, in which a carbon chain or carbon ring is expanded by a one-carbon unit, have been widely used in complex molecule synthesis25,26,27. Among various types of ring-expansion reactions, the coupling of cyclic ketones with diazoalkanes, namely the Büchner-Curtius-Schlotterbeck reaction, has been intensively investigated28,29,30. In 2008, an important breakthrough was achieved in this regard by Davies, who demonstrated an elegant protocol on the rhodium-catalyzed ring expansion of isoxazoles via rhodium carbene intermediates insertion into the N−O bond of isoxazoles (Fig. 1a)31. Since then, a variety of reactions in which single carbon atom is inserted into the N–O bond of heterocyclic compounds have been reported32,33,34,35. However, these protocols have to rely on the use of hazardous and not easily accessible diazo compounds as precursors, which severely limit their further synthetic applications and the molecular flexibility. Moreover, to our knowledge, examples of the relevant asymmetric catalysis have not been reported. Thus, it is highly desirable to develop new methods for one-carbon ring expansion, especially those with high flexibility, efficiency, and stereoselectivity.
In the past decade, the chemistry of vinyl cations has received particular attention because of their unique carbene-like reactivity36,37. Recently, our group reported a copper-catalyzed diyne cyclization via vinyl cations as key intermediates, providing a variety of polycyclic pyrrole derivatives38,39,40,41,42,43,44,45,46,47. In particular, the related catalytic asymmetric transformations were established via a remote control of enantioselectivity. Inspired by these results and by our recent study of the development of ynamide chemistry for heterocycle synthesis48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65, we envisioned that the vinyl cations generated from copper-catalyzed diyne cyclization might be trapped by 1,2,5-oxadiazoles, eventually leading to valuable 1,2,5-oxadiazines via one-carbon homologation (Fig. 1b). Herein, we describe such a copper-catalyzed one-carbon ring expansion of 1,2,5-oxadiazoles through vinyl cation intermediates generated from N-propargyl ynamides, thus constituting an intermolecular formal (5 + 1) annulation of 1,5-diynes with 1,2,5-oxadiazoles.
Results and discussion
Screening of reaction conditions
To prohibit the background C–H insertion reaction38, 2,6-dimethylphenyl-substituted N-propargyl ynamide 1a was first chosen as the model substrate to react with 1,2,5-oxadiazole 2a under our previous related reaction conditions38,39,40,41,42,43,44,45,46,47, and selected results are listed in Table 1. To our delight, the expected 1,2,5-oxadiazine 3a was indeed formed in 37% yield in the presence of 10 mol % of CuOTf as catalyst (Table 1, entry 1). Subsequent screening of other copper catalysts (Table 1, entries 2–3) revealed that Cu(MeCN)4PF6 was the best catalyst to deliver the desired product 3a in 53% yield (Table 1, entry 3). In addition, the use of other typical solvents such as DCM, CHCl3, PhMe and PhCl led to decreased yields (Table 1, entries 4–7). Of note, the formation of byproducts decreased with the increase of the equiv of 2a, and 5 equiv of 2a was found to be the most appropriate (Table 1, entries 8–9). Gratifyingly, the reaction could be significantly promoted by the addition of 12 mol % of NaBArF4 (Table 1, entry 10), and the yield of the reaction could be further increased to 72% under nitrogen atmosphere (Table 1, entry 11). Of note, it was found that the temperature had very slight impact on the yield of 3a (Table 1, entries 12–13).
Reaction scope study
With the optimized reaction conditions in hand (Table 1, entry 11), the scope of this copper-catalyzed formal (5 + 1) annulation was explored. As depicted in Fig. 2, in general, ynamides with different N-protecting groups, such as Ns, Ts, Bs and MBS groups, could proceed smoothly to provide the corresponding 1,2,5-oxadiazines 3a–3d in 61–72% yields (see Supplementary Data 1, 2). In addition, various aryl-substituted ynamides (R1 = Ar) bearing both electron-donating and -withdrawing groups on the aromatic ring were tolerated for this reaction, leading to the expected products 3e–3i in 64–76% yields. Moreover, the reaction occurred efficiently for a variety of aryl-substituted N-propargyl ynamides (R2 = Ar), producing the target oxadiazines 3j–3o in 55–84% yields. Interestingly, 2-thienyl-substituted and alkenyl-substituted ynamides were also suitable substrates to deliver the desired products 3p (78%) and 3q (57%), respectively. Of note, 2-methylphenyl-substituted N-propargyl ynamide 1r was also tolerated to afford the expected 3r in 61% yield while the use of phenyl-substituted N-propargyl ynamide 1 s only led to complicated mixtures. Moreover, the cyclopropyl-substituted ynamide could also be smoothly converted into the expected product 3t in 51% yield. Particularly, this formal (5 + 1) annulation was also extended to other aryl-substituted 1,2,5-oxadiazoles, allowing the formation of the corresponding products 3u–3 v in 62–73% yields. Finally, the methyl-substituted 1,2,5-oxadiazole was tolerated in this reaction, and the expected product 3w was formed in 56% yield. The structure of product 3a was further confirmed by X-ray diffraction analysis (Fig. 3). Thus, this protocol provides a unique way for rapid and efficient assembly of 1,2,5-oxadiazine derivatives, which are not readily accessible by known methods. It is notable that the use of the alkyl-substituted ynamide 1 y (R2 = alkyl) as substrate and the relevant thiadiazole and triazole as nucleophiles only led to complicated mixtures under the optimal and related conditions.
Inspired by the above results, we then explored the chiral copper-catalyzed asymmetric formal (5 + 1) annulation. Although direct asymmetric catalysis based on the reaction of N-propargyl ynamide 1a and 1,2,5-oxadiazole 2a failed to give promising enantioselectivity (<20% ee), the use of the steric group-substituted N-propargyl ynamide 1x as substrate could lead to moderate enantioselectivity bases on our recent study on the chiral copper-catalyzed atroposelective diyne cyclization45. As depicted in Fig. 4, we were pleased to find that the use of bisoxazoline (BOX) ligand L* as the chiral ligand resulted in the formation of the desired chiral oxadiazine 3x in 57% yield and 4:1 d.r. with 60% ee.
Synthetic applications
To further demonstrate the utility of this annulation reaction, we carried out several synthetic transformations of the pyrrole-substituted oxadiazine 3a, as illustrated in Fig. 5. First, the preparative-scale reaction of ynamide 1a was conducted under the standard conditions, and the desired product 3a was formed in 72% yield. In addition, the Ns group of 3a could be readily removed to deliver product 4a in 77% yield. Interestingly, the treatment of 4a with 5 equiv of KOH led to the formation of the corresponding N-oxide compound 5a in 95% yield via a ring contraction way.
Plausible reaction mechanism
On the basis of the above experimental results and our previous study on the copper-catalyzed cyclization of N-propargyl ynamides38,39,40,41,42,43,44,45,46,47, a plausible reaction mechanism to rationalize the formation of pyrrole-substituted oxadiazine 3a is shown in Fig. 6. First, catalytic Cu(I) species coordinate with the electron-richer amide-tethered C–C triple bond of ynamide 1a, generating the precursor A, which subsequently undergoes nucleophilic attack by another C−C triple bond to produce the key vinyl cation intermediate B. Then, the vinyl cation is trapped by oxadiazole 2a, providing intermediate C. Next, ring-opening of oxadiazole, followed by 6π electrocyclization, leads to donor/donor copper carbene intermediate E. Finally, intermediate E undergoes [1,4]-H shift and demetallation to furnish the corresponding product 3a. On the other hand, by using chiral copper catalyst, chiral product 3x can be obtained via asymmetric atroposelective cyclization through a remote control of enantioselectivity.
Conclusions
In summary, we have disclosed a copper-catalyzed intermolecular formal (5 + 1) annulation of 1,5-diynes with 1,2,5-oxadiazoles, allowing the practical and atom-economic synthesis of valuable 1,2,5-oxadiazine derivatives in generally moderate to good yields under mild reaction conditions. This reaction is realized by inserting vinyl cations formed through 1,5-diyne cyclization into the N–O bond of 1,2,5-oxadiazoles. In addition, the possibility of such an asymmetric formal (5 + 1) annulation also emerges. Importantly, the protocol achieves one-carbon ring expansion of 1,2,5-oxadiazoles base on vinyl cations, and provides a rare approach to 1,2,5-oxadiazines. Further application of this type of copper-catalyzed one-carbon homologation will be pursued in our laboratory.
Methods
Materials
Unless otherwise noted, materials were obtained commercially and used without further purification. All the solvents were treated according to general methods. Flash column chromatography was performed over silica gel (300–400 mesh). See Supplementary Methods for experimental details.
General methods
1H NMR spectra and 13C NMR spectra were recorded on a Bruker AV-400 spectrometer in chloroform-d3. For 1H NMR spectra, chemical shifts are reported in ppm with the internal TMS signal at 0.0 ppm as a standard. For 13C NMR spectra, chemical shifts are reported in ppm with the internal chloroform signal at 77.0 ppm as a standard. Infrared spectra were recorded on a Nicolet AVATER FTIR330 spectrometer as thin film and are reported in reciprocal centimeter (cm−1). Mass spectra were recorded with Micromass QTOF2 Quadrupole/Time-of-Flight Tandem mass spectrometer using electron spray ionization. 1H NMR, 13C NMR spectra and HPLC spectra are supplied for all compounds: see Supplementary Information. See Supplementary Methods for the characterization data of compounds not listed in this part.
General procedure for the synthesis of 1,2,5-oxadiazines 3
1,2,5-oxadiazole 2 (0.5 mmol), NaBArF4 (0.012 mmol, 2.7 mg), and Cu(MeCN)4PF6 (0.01 mmol, 3.7 mg) were added in this order to the N-propargyl ynamide 1 (0.1 mmol) in DCE (2 mL) at room temperature. The reaction mixture was stirred at 40 °C and the progress of the reaction was monitored by TLC. Upon completion, the mixture was concentrated under reduced pressure and the residue was purified by column chromatography on silica gel (dichloromethane/hexane) to afford the desired product 3.
Data availability
Data for the crystal structure reported in this paper has been deposited at the Cambridge Crystallographic Data Centre (CCDC) under the deposition number CCDC 2268060 (3a). Copies of these data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif. All other data supporting the findings of this study, including experimental procedures and compound characterization, are available within the paper and its Supplementary Data 1, 2, or from the corresponding authors on request.
References
Omar, A.-M. M. E., AboulWafa, O. M., Amr, M. E. & El-Shoukrofy, M. S. Antiproliferative activity, enzymatic inhibition and apoptosis-promoting effects of benzoxazole-based hybrids on human breast cancer cells. Bioorg. Chem. 109, 104752 (2021).
Zhang, J., Hao, W., Zhorov, B. S., Dong, K. & Jiang, D. Discovery of a novel series of tricyclic oxadiazine 4a-methyl esters based on indoxacarb as potential sodium channel blocker/modulator insecticides. J. Agric. Food Chem. 67, 7793–7809 (2019).
Hashem, H. E. & Abo-Bakr, A. M. Synthesis of some new 1,2,4-triazine and 1,2,5-oxadiazine derivatives with antimicrobial activity. Heteroat. Chem. 2019, 1–7 (2019).
Eissa, F. M. Green synthesis, antibacterial, and antifungal activities of 1,3,4-oxadiazines. J. Heterocyclic Chem. 55, 1479–1483 (2018).
Bursavich, M. G. et al. Design, synthesis, and evaluation of a novel series of oxadiazine gamma secretase modulators for familial Alzheimer’s disease. J. Med. Chem. 60, 2383–2400 (2017).
Lee, J. et al. Discovery of highly selective and potent monoamine oxidase B inhibitors: contribution of additional phenyl rings introduced into 2-aryl-1,3,4-oxadiazin-5(6H)-one. Eur. J. Med. Chem. 130, 365–378 (2017).
Berthet, M., Legrand, B., Martinez, J. & Parrot, I. A general approach to the aza-diketomorpholine scaffold. Org. Lett. 19, 492–495 (2017).
Duan, Z. & Shao, L. Synthesis and antimicrobial activity of flavone derivatives containing 1,3,4-oxadiazoline structure. Chin. J. Org. Chem. 35, 2004–2012 (2015).
Carreira, E. M. & Fessard, T. C. Four-membered ring-containing spirocycles: synthetic strategies and opportunities. Chem. Rev. 114, 8257–8322 (2014).
Majumdar, P., Pati, A., Patra, M., Behera, R. K. & Behera, A. K. Acid hydrazides, potent reagents for synthesis of oxygen-, nitrogen-, and/or sulfur-containing heterocyclic rings. Chem. Rev. 114, 2942–2977 (2014).
Borthwick, A. D. 2,5-Diketopiperazines: synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 112, 3641–3716 (2012).
Ke, S., Cao, X., Liang, Y., Wang, K. & Yang, Z. Synthesis and biological properties of dihydro-oxadiazine-based heterocyclic derivatives. Mini-Rev. Med. Chem. 11, 642–657 (2011).
Bigot, A., Blythe, J., Pandya, C., Wagner, T. & Loiseleur, O. DAST-mediated cyclization of α,α-disubstituted-α-acylaminoketones: efficient and divergent synthesis of unprecedented heterocycles. Org. Lett. 13, 192–195 (2011).
Erian, A. W. Recent trends in the chemistry of fluorinated five and six-membered heterocycles. J. Heterocyclic Chem. 38, 793–808 (2001).
Mishra, M., Maharana, P. K., Karjee, P. & Punniyamurthy, T. Expedient cobalt-catalyzed stereospecific cascade C–N and C–O bond formation of styrene oxides with hydrazones. Chem. Commun. 58, 7090–7093 (2022).
Zhang, Y., Kuang, J., Xiao, X., Wang, L. & Ma, Y. DMSO as a dual carbon synthon and water as oxygen donor for the construction of 1,3,5-oxadiazines from amidines. Org. Lett. 23, 3960–3964 (2021).
Guo, X. et al. Organocatalytic enantioselective [2+4] annulation of γ-substituted allenoates with N-acyldiazenes for the synthesis of optically active 1,3,4-oxadiazines. Org. Biomol. Chem. 19, 1727–1731 (2021).
Li, M., Li, W., Lin, C.-D., Wang, J.-H. & Wen, L.-R. One base for two shots: metal-free substituent-controlled synthesis of two kinds of oxadiazine derivatives from alkynylbenziodoxolones and amidoximes. J. Org. Chem. 84, 6904–6915 (2019).
Kuruba, B. K. & Vasanthkumar, S. An efficient protocol for the synthesis of six-membered N,O-heterocycles via a 1,3-dipolar (3+3) cycloaddition between nitrile oxide and α-diazo esters. Tetrahedron 73, 3860–3865 (2017).
Zhang, Q., Meng, L.-G., Zhang, J. & Wang, L. DMAP-catalyzed [2+4] cycloadditions of allenoates with N-acyldiazenes: direct method to 1,3,4-oxadiazine derivatives. Org. Lett. 17, 3272–3275 (2015).
Morrill, L. C., Lebl, T., Slawin, A. M. Z. & Smith, A. D. Catalytic asymmetric α-amination of carboxylic acids using isothioureas. Chem. Sci. 3, 2088–2093 (2012).
Huang, X.-L., He, L., Shao, P.-L. & Ye, S. [4+2] Cycloaddition of ketenes with N-benzoyldiazenes catalyzed by N-heterocyclic carbenes. Angew. Chem. Int. Ed. 48, 192–195 (2009).
Cho, S. Y., Kang, S. K., Ahn, J. H., Ha, J. D. & Choi, J.-K. Scandium(III) triflate-TMSCl promoted cyclization of aziridin-1-yl oximes to 5,6-dihydro-4H-[1,2,4]oxadiazines. Tetrahedron Lett 47, 9029–9033 (2006).
Yang, L. et al. Asymmetric NHC-catalyzed aza-Diels−Alder reactions: highly enantioselective route to α-amino acid derivatives and DFT calculations. Org. Lett. 16, 3872–3875 (2014).
Pace, V. Homologation reactions reagents, applications, and mechanisms. Wiley-VCH, Weinheim (2022).
Ochi, S., Zhang, Z., Xia, Y. & Dong, G. Rhodium-catalyzed (4+1) cycloaddition between benzocyclobutenones and styrene-type alkenes. Angew. Chem. Int. Ed. 61, e202202703 (2022).
Kamitani, M. et al. Single–carbon atom transfer to α,β-unsaturated amides from N-heterocyclic carbenes. Science 379, 484–488 (2023).
Leemans, E., D’hooghe, M. & Kimpe, N. D. Ring expansion of cyclobutylmethylcarbenium ions to cyclopentane or cyclopentene derivatives and metal-promoted analogous rearrangements. Chem. Rev. 111, 3268–3333 (2011).
Candeias, N. R., Paterna, R. & Gois, P. M. P. Homologation reaction of ketones with diazo compounds. Chem. Rev. 116, 2937–2981 (2016).
Moebius, D. C., Rendina, V. L. & Kingsbury, J. S. Catalysis of diazoalkane–carbonyl homologation. How new developments in hydrazone oxidation enable the carbon insertion strategy for synthesis. Top. Curr. Chem 346, 111–162 (2014).
Manning, J. R. & Davies, H. M. L. Efficient route to 2H-1,3-oxazines through ring expansion of isoxazoles by rhodium carbenoids. Tetrahedron 64, 6901–6908 (2008).
Khlebnikov, A. F. et al. Isoxazolium N-ylides and 1-oxa-5-azahexa-1,3,5-trienes on the way from isoxazoles to 2H-1,3-oxazines. Beilstein J. Org. Chem. 10, 1896–1905 (2014).
Jurberg, I. D. & Davies, H. M. L. Rhodium- and non-metal-catalyzed approaches for the conversion of isoxazol-5-ones to 2,3-dihydro-6H-1,3-oxazin-6-ones. Org. Lett. 19, 5158–5161 (2017).
Han, C., Wu, W., Chen, Z. & Pu, S. Rhodium-catalyzed [5+1]-cycloaddition reactions to spiro-benzo[e][1,3]oxazineindoline imines. Asian J. Org. Chem. 8, 1385–1389 (2019).
Strelnikova, J. O., Rostovskii, N. V., Khoroshilova, O. V., Khlebnikov, A. F. & Novikov, M. S. An efficient synthesis of functionalized 2H-1,3,5-oxadiazines via metal-carbenoid-induced 1,2,4-oxadiazole ring cleavage. Synthesis 53, 348–358 (2021).
Niggemann, M. & Gao, S. Are vinyl cations finally coming of age? Angew. Chem. Int. Ed. 57, 16942–16944 (2018).
Liu, X.-J., Xu, Y., Tang, C., Qian, P.-C. & Ye, L.-W. Unactivated C(sp3)–H functionalization via vinyl cations. Sci. China: Chem. 65, 20–30 (2022).
Hong, F.-L. et al. Generation of donor/donor copper carbenes through copper-catalyzed diyne cyclization: enantioselective and divergent synthesis of chiral polycyclic pyrroles. J. Am. Chem. Soc. 141, 16961–16970 (2019).
Hong, F.-L. et al. Copper-catalyzed asymmetric reaction of alkenyl diynes with styrenes by formal [3+2] cycloaddition via Cu-containing all-carbon 1,3-dipoles: access to chiral pyrrole-fused bridged [2.2.1] skeletons. J. Am. Chem. Soc. 142, 7618–7626 (2020).
Zhu, X.-Q. et al. Copper-catalyzed asymmetric cyclization of alkenyl diynes: method development and new mechanistic insights. Chem. Sci. 12, 9466–9474 (2021).
Zhu, G.-Y. et al. Copper-catalyzed cyclization of N-propargyl ynamides with borane adducts through B−H bond insertion. Org. Lett. 23, 8067–8071 (2021).
Hong, F.-L. et al. Copper-catalyzed asymmetric diyne cyclization via [1,2]-Stevens-type rearrangement for the synthesis of chiral chromeno[3,4-c]pyrroles. Angew. Chem. Int. Ed. 61, e202115554 (2022).
Qi, L.-J. et al. Enantioselective copper-catalyzed formal [2+1] and [4+1] annulations of diynes with ketones via carbonyl ylides. Angew. Chem. Int. Ed. 61, e202210637 (2022).
Huang, E.-H. et al. Copper-catalyzed Si−H bond insertion reaction of N-propargyl ynamides with hydrosilanes. Org. Lett. 24, 196–201 (2022).
Chen, Y.-B. et al. Construction of axially chiral arylpyrroles via atroposelective diyne cyclization. Angew. Chem. Int. Ed. 62, e202303670 (2023).
Zhou, J.-J. et al. Copper-catalyzed enantioselective diyne cyclization via C(sp2)–O bond cleavage. Chem. Sci. 14, 3493–3500 (2023).
Xu, H.-J. et al. Copper-catalyzed formal [4+1] annulation of N-propargyl ynamides with diketones. Org. Chem. Front. 10, 203–208 (2023).
Hu, Y.-C., Zhao, Y., Wan, B. & Chen, Q.-A. Reactivity of ynamides in catalytic intermolecular annulations. Chem. Soc. Rev. 50, 2582–2625 (2021).
Chen, Y.-B., Qian, P.-C. & Ye, L.-W. Brønsted acid-mediated reactions of ynamides. Chem. Soc. Rev. 49, 8897–8909 (2020).
Lynch, C. C., Sripada, A. & Wolf, C. Asymmetric synthesis with ynamides: unique reaction control, chemical diversity and applications. Chem. Soc. Rev. 49, 8543–8583 (2020).
Hong, F.-L. & Ye, L.-W. Transition metal-catalyzed tandem reactions of ynamides for divergent N-heterocycle synthesis. Acc. Chem. Res. 53, 2003–2019 (2020).
Zhou, B., Tan, T.-D., Zhu, X.-Q., Shang, M. & Ye, L.-W. Reversal of regioselectivity in ynamide chemistry. ACS Catal 9, 6393–6406 (2019).
Evano, G., Theunissen, C. & Lecomte, M. Ynamides: powerful and versatile reagents for chemical synthesis. Aldrichimica Acta 48, 59–70 (2015).
Wang, X.-N. et al. Ynamides in ring forming transformations. Acc. Chem. Res. 47, 560–578 (2014).
Liu, X. et al. Copper-catalyzed enantioselective Doyle–Kirmse reaction of azide-ynamides via α-imino copper carbenes. Angew. Chem. Int. Ed. 62, e202216923 (2023).
Li, H.-H. et al. Chiral Brønsted acid-catalyzed asymmetric intermolecular [4+2] annulation of ynamides with para-quinone methides. Sci. China Chem. 66, 1467–1473 (2023).
Zhang, Z.-X. et al. Brønsted acid-catalyzed asymmetric dearomatization of indolyl ynamides: practical and enantioselective synthesis of polycyclic indolines. Chin. Chem. Lett. 34, 107647 (2023).
Zhu, G.-Y. et al. Catalyst-dependent stereospecific [3,3]-sigmatropic rearrangement of sulfoxide-ynamides: divergent synthesis of chiral medium-sized N,S-heterocycles. Angew. Chem. Int. Ed. 61, e202204603 (2022).
Wang, Z.-S. et al. Synthesis of axially chiral N-arylindoles via atroposelective cyclization of ynamides catalyzed by chiral Brønsted acids. Angew. Chem. Int. Ed. 61, e202201436 (2022).
Li, H.-H. et al. Metal-free dearomatization reactions of naphtholynamides for the divergent and enantioselective synthesis of azaspirocycles. Org. Chem. Front. 9, 3709–3717 (2022).
Zhang, Y.-Q. et al. Asymmetric dearomatization catalyzed by chiral Brønsted acids via activation of ynamides. Nat. Chem. 13, 1093–1100 (2021).
Chen, P.-F., Zhou, B., Wu, P., Wang, B. & Ye, L.-W. Brønsted acid catalyzed dearomatization by intramolecular hydroalkoxylation/claisen rearrangement: diastereo- and enantioselective synthesis of spirolactams. Angew. Chem. Int. Ed. 60, 27164–27170 (2021).
Zhang, Y.-Q., Zhang, Y.-P., Zheng, Y.-X., Li, Z.-Y. & Ye, L.-W. Rapid and practical access to diverse quindolines by catalyst-free and regioselectivity-reversed Povarov reaction. Cell Rep. Phy. Sci. 2, 100448–100462 (2021).
Wang, Z.-S. et al. Ynamide smiles rearrangement triggered by visible-light-mediated regioselective ketyl-ynamide coupling: rapid access to functionalized indoles and isoquinolines. J. Am. Chem. Soc. 142, 3636–3644 (2020).
Liu, X. et al. Copper-catalyzed azide-ynamide cyclization to generate α-imino copper carbenes: divergent and enantioselective access to polycyclic N-heterocycles. Angew. Chem. Int. Ed. 59, 17984–17990 (2020).
Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (22125108 and 92056104), the President Research Funds from Xiamen University (20720210002), and NFFTBS (J1310024). We thank Mr. Zanbin Wei from Xiamen University (College of Chemistry and Chemical Engineering) for assistance with X-ray crystallographic analysis.
Author information
Authors and Affiliations
Contributions
C.-M.C., Y.-N.Y., Y.-Z.K., B.-H.Z., and B.Z. performed experiments. L.-W.Y. and P.-C.Q conceived and directed the project and wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks Thierry Ollevier and the other anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, CM., Yang, YN., Kong, YZ. et al. Copper-catalyzed intermolecular formal (5 + 1) annulation of 1,5-diynes with 1,2,5-oxadiazoles. Commun Chem 6, 194 (2023). https://doi.org/10.1038/s42004-023-00999-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-023-00999-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.