Abstract
Nitrile derivatives are important building blocks in organic synthesis. Herein, we report the serendipitous discovery of an oxygen transfer reaction that produces hydroxyalkyl nitriles from the sequential dehydration and hydrolysis of haloalkyl amides. Product yields of up to 91% were achieved, and the phenylboronic acid was recovered as triphenylboroxine. The triphenylboroxine was reused as a catalyst without any loss of catalytic activity. A probable catalytic pathway was proposed based on control experiments and DFT calculations.
Similar content being viewed by others
Introduction
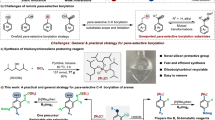
Organonitrile compounds are important to synthesis in medicinal, biological, and materials chemistry1,2,3,4,5 because of the very unique reactivity and activating ability of the nitrile group6,7,8. There are several methods for the synthesis of nitrile derivatives9,10,11,12,13, which are mainly based on the transition-metal-catalyzed reaction of aryl/alkyl halides or alcohols14,15,16,17,18,19,20,21. In addition, amide dehydration is an important method for the construction of the nitrile moiety22,23,24. Previously, the dehydration of a primary amide was conducted using harsh and acidic dehydrating reagents such as POCl322, P4O1023, and SOCl224 (Fig. 1a). Recently, there has been significant interest in the dehydration of amides25,26,27,28,29,30,31,32,33 catalyzed by transition metals such as Pd26,27,28,29, Re30, Fe32, In31 and Cu33 (Fig. 1b). On the other hand, the hydrolysis of alkyl halides to generate the corresponding alcohols is a textbook reaction34,35,36. Hydroxyl and nitrile groups are important because both of these functionalities can be found in several biologically active molecules and naturally occurring substrates5,37,38,39,40,41.
A domino conversion of amide to nitrile and alkyl halide to the corresponding alcohol would be a very straightforward strategy for the construction of hydroxyalkyl nitriles (Fig. 1c). Herein, we report oxygen transfer reaction of haloalkyl amides affording hydroxyalkyl nitriles using a metal-free catalyst, phenylboronic acid, which can be reused without loss of catalytic activity.
Results and discussion
Reaction discovery
We previously reported the formation of lactams, phenanthridinone (3) by intramolecular amidation of aryl halides and amides using a polymeric nickel catalyst and phenylboronic acid (Fig. 2a)42. However, the reaction of 6-bromohexanamide (4a) surprisingly produced 6-hydroxyhexanenitrile (5a) in 80% yield instead of lactam 6 (Fig. 2b). Eventually, phenylboronic acid (2a) became the catalyst43,44,45,46,47,48,49 in this transformation, and the oxygen transfer reaction of 4a with 20 mol% of 2a and 3 molar equiv of potassium phosphate gave 5a in 80% yield (Fig. 2c and Table 1, entry 1).
The effects of deviation from the standard reaction conditions are shown in Tables 1 and S1 (Supporting Information). The use of 20 mol% of 2-phenyl-1,3,2-dioxaborinane (7) as the catalyst gave 72% of 5a (entry 2), which was similar as the yield of the reference reaction (80%, entry 1). The reaction afforded 60% of 5a when the amount of 2a was reduced from 20 to 10 mol% (entry 3). In the absence of 2a, no reaction occurred, confirming that this compound is the catalyst in this transformation (entry 4). The reaction in toluene as the solvent afforded 5a in 72% yield (entry 5). When a stronger base, potassium tert-butoxide (KOtBu), was used, the desired product 5a was not obtained (entry 6), and the simple β-elimination of the terminal alkyl bromide moiety occurred instead (see Supporting Information for more details).
Substrate scope
With the optimized reaction conditions in hand, we examined the substrate scope of this reaction (Fig. 3). The reaction of 4a produced 5a in 80% yield. The reactions of 6-chlorohexanamide (4a’) and 6-iodohexanamide (4a”) also afforded 5a in 47 and 74% yield, respectively. The reaction of 7-bromoheptanamide furnished 7-hydroxyheptanenitrile (5b) in 39% yield. The conversion of 5-bromopentanamide (4c) afforded 5-hydroxypentanenitrile (5c) in higher yield (86%). The reaction of the oxygen-tethered 2-(2-bromoethoxy)acetamide (4d) afforded 5d in 75% yield. Similarly, 4-bromobutanamide (4e) provided 5e in 60% yield. Unfortunately, the reaction of 3-bromopropanamide (4f) did not proceed. Here acrylamide was observed as the major product (see Supporting Information for more details). The reaction of a secondary alkyl bromide, 5-bromohexanamide (4g), showed the best result, providing 5g in 91% yield. Moreover, a secondary alkyl chloride, 5-chlorohexanamide (4g’), was also converted to 5g in 82% yield. The reaction of the cyclic compound, 2-(bromomethyl)cyclohexane-1-carboxamide (4h), proceeded to afford 5h in 63% yield. The yield of 5h was determined after tosylation (see Supporting Information for more details). The reaction of an aromatic amide (4i) was unsuccessful where lactam was obtained as a major product.
Catalyst reusability
The reusability of the catalyst was then investigated (Fig. 4a, b). After the reaction, the catalyst was recovered as a cyclic trimer (phenylboroxine, 8) in 86% yield through column chromatographic separation (Fig. 4a). When the recovered 8 was used as the catalyst, the reaction proceeded efficiently to provide 81% yield of 5a (Fig. 4b).
a Catalyst recovery. b Reusability of recovered catalyst 8. c Dehydration of amide without halogen tethering. d Hydrolysis of secondary amide under standard reaction conditions. e Intermolecular competition with/without halogen tethering. f Intermolecular competition between primary and secondary amide. g The effect of molecular sieves. h the effect of water. i The effect of 18O-water. j The effect of 20 mol% of Ph-B(18OH)2. k the effect of 1.5 molar equiv of Ph-B(18OH)2.
Control experiments
The reaction of 4j (a halogen-free substrate, Fig. 4c) and 9 (a secondary amide with no possibility of water generation, Fig. 4d) were not converted to the corresponding nitriles and alcohols, respectively. The reaction of 4a proceeded to afford 5a in the presence of 4j (Fig. 4e) and 9 (Fig. 4f) whereas the corresponding nitrile (5j) and alcohol (10) were not observed. The yield of 5a was dropped slightly to 71% when molecular sieves (4A) were added to the reaction mixture as dehydrating agents (Fig. 4g). Contrarily, the yield of 5a was neither increased nor decreased in presence of one mol equiv of water (Fig. 4h). The addition of 18O-water produced a trace amount of 18O-5a product (Fig. 4i) which suggests water is not directly involved in this reaction but it could take part via the equilibrium between phenylboronic acid and its corresponding anhydride50. Contrarily, the amount of 18O-5a was increased significantly (5a:18O-5a = 9:1) when 18O-2a (16O2:16O18O:18O2 = 14:23:64)51 was used as catalyst (Fig. 4j).In addition, 18O-5a was observed as the main product(5a:18O-5a = 1:3) when 150 mol% (1.5 molar equiv) of 18O-2a was used as additive (Fig. 4k). This increase of 18O in the product suggest the oxygen transfer proceeded via the phenylboronic acid.
Plausible catalytic pathway
A plausible catalytic pathway is proposed in Fig. 5. Initially, the amide substrates (4) present in a equilibrium with the corresponding imidic acid intermediate (11). Next, the base (K3PO4) deprotonate the intermediate 11 to form the intermediate 12. Next, intermediate 12 reacts with phenylboronic acid (2a) to form intermediate A. The hydroxyl group attached with boron acts as a nucleophile and substitutes the halide ion via nucleophilic substitutuion reaction to form intermediate B. Finally, a carbon-nitrogen triple (nitrile) bond formation regenerates the phenylboronic acid (2a) and affoard the product (5). Phenylboroxine (8) might generate after the end of the catalytic cycle.
DFT studies
To support our proposed reaction mechanism, we performed DFT calculations (see the Supporting Information and Supplementary Figs. 1 and 2). As shown in Fig. 6, our calculations suggested that the reaction proceeds through a two-step mechanism. First, a deprotonated amide substrate interacts with boronic acid 2a to form a tetrahedral boron intermediate A. An intramolecular nucleophilic substitution of the bromine with one OH group then occurs via the transition state TS1, which results in the intermediate B. The transition state was calculated to be 21.0 kcal/mol higher in energy compared to intermediate A. The second step is the cleavage of the C–O bond and formation of carbon-nitrogen triple bond. We found that K2HPO4 plays an essential role in the second step. One oxygen atom of K2HPO4 is coordinated to the boron atom in intermediate B’ where NH and POH protons are directed towards the PO and CO oxygen atoms, respectively. The NH and POH protons simultaneously transfer through a single transition state (TS2) to give the corresponding hydroxyalkyl nitrile (intermediate C). The activation energy for TS2 (26.7 kcal/mol) was slightly higher than that of TS1.
Conclusion
In summary, we found an oxygen transfer reaction during the sequential dehydration of primary alkyl amides and hydrolysis of alkyl halides to afford hydroxyalkyl nitriles, which was catalyzed by phenylboronic acid. In this reaction, a wide variety of substrates were tolerated and the catalyst could be recovered and reused. The reaction pathway for this unique transformation was proposed based on DFT calculations and control experiments. Complete mechanistic studies are now ongoing in our laboratory.
Methods
General procedure for the synthesis of hydroxynitrile derivatives. A mixture of phenylboronic acid 2a (20 mol%, 24.2 mg), an amide 4 (1 mol equiv, 1 mmol), and K3PO4 (3 mol equiv, 636 mg) was added to a reaction tube (see supplementary methods). The reaction tube was degassed under vacuum and refilled with N2 under standard Schlenk techniques (3 times). To the reaction mixture, 1,4-dioxane was added (2 mL). The reaction tube was sealed with a screw cap and teflon and then stirred at 115 °C under nitrogen for 72 h. Finally, the reaction was quenched with 1 N HCl, and the reaction mixture was extracted with EtOAc. The EtOAc layer was collected and dried over Na2SO4. The solvent was evaporated under vacuum, and the resulting crude mixture was purified by column chromatography (hexane/ethyl acetate) to give product 5.
Data availability
All the data created for this studies (compound characterization, 1H-NMR, 13C-NMR, HRMS, melting point) are present in supporting information file (PDF), the cartesian coordinates for the DFT studies are shown in Supplementary Data 1 File (PDF), the NMR and GC spectras are shown in Supplementary Data 2 File (PDF).
Change history
22 February 2023
A Correction to this paper has been published: https://doi.org/10.1038/s42004-023-00842-4
References
Patai, S. The Chemistry of the Cyano Group (ed Rappoport, Z.) 717–742 (Wiley, 1971).
Gregory, R. J. H. Cyanohydrins in nature and the laboratory: biology, preparations, and synthetic applications. Chem. Rev. 99, 3649–3682 (1999).
Miller, J. S. & Manson, J. L. Designer magnets containing cyanides and nitriles. Acc. Chem. Res. 34, 563–570 (2001).
Fleming, F. F. & Wang, Q. Unsaturated nitriles: conjugate additions of carbon nucleophiles to a recalcitrant class of acceptors. Chem. Rev. 103, 2035–2077 (2003).
Fleming, F. F., Yao, L., Ravikumar, P. C., Funk, L. & Shook, B. C. Nitrile-containing pharmaceuticals: efficacious roles of the nitrile pharmacophore. J. Med. Chem. 53, 7902–7917 (2010).
Comprehensive Organic Functional Group Transformations II 2nd edn (eds Katrizky, A. R., Taylor, R. J. K.) Vol. 2 (Elsevier, 2004).
Subramanian, L. R. Nitriles. in science of synthesis, Trost, B. M., Lautens, M., eds. Thieme Verl.: Stuttg. 19, 79 (2011).
Vanjari, R., Dutta, S., Gogoi, M. P., Gandon, V. & Sahoo, A. K. Gold-catalyzed syn-1,2-difunctionalization of ynamides via nitrile activation. Org. Lett. 20, 8077–8081 (2018).
Czekelius, C. & Carreira, E. M. Convenient transformation of optically active nitroalkanes into chiral aldoximes and nitriles. Angew. Chem. Int. Ed. Engl. 44, 612–615 (2005).
Nicolaou, K. C. & Mathison, C. J. N. Synthesis of imides, N-acyl vinylogous carbamates and ureas, and nitriles by oxidation of amides and amines with Dess–Martin periodinane. Angew. Chem. Int. Ed. Engl. 44, 5992–5997 (2005).
Laulhé, S., Gori, S. S. & Nantz, M. H. A chemoselective, one-pot transformation of aldehydes to nitriles. J. Org. Chem. 77, 9334–9337 (2012).
Yu, L. et al. Organoselenium-catalyzed mild dehydration of aldoximes: an unexpected practical method for organonitrile synthesis. Org. Lett. 16, 1346–1349 (2014).
McManus, J. B. & Nicewicz, D. A. Direct C–H cyanation of arenes via organic photoredox catalysis. J. Am. Chem. Soc. 139, 2880–2883 (2017).
Zanon, J., Klapars, A. & Buchwald, S. L. Copper-catalyzed domino halide exchange-cyanation of aryl bromides. J. Am. Chem. Soc. 125, 2890–2891 (2003).
Liskey, C. W., Liao, X. & Hartwig, J. F. Cyanation of arenes via iridium-catalyzed borylation. J. Am. Chem. Soc. 132, 11389–11391 (2010).
Yin, W., Wang, C. & Huang, Y. Highly practical synthesis of nitriles and heterocycles from alcohols under mild conditions by aerobic double dehydrogenative Catalysis. Org. Lett. 15, 1850–1853 (2013).
Dornan, L. M. et al. Copper/TEMPO catalysed synthesis of nitriles from aldehydes or alcohols using aqueous ammonia and with air as the oxidant. Chem. Commun. 49, 6030–6032 (2013).
Shen, T., Wang, T., Qin, C. & Jiao, N. Silver-catalyzed nitrogenation of alkynes: a direct approach to nitriles through C≡C bond cleavage. Angew. Chem. Int. Ed. 52, 6677–6680 (2013).
Jagadeesh, R. V., Junge, H. & Beller, M. Green synthesis of nitriles using non-noble metal oxides-based nanocatalysts. Nat. Commun. 5, 4123 (2014).
Wu, Q., Luo, Y., Lei, A. & You, J. Aerobic copper-promoted radical-type cleavage of coordinated cyanide anion: nitrogen transfer to aldehydes to form nitriles. J. Am. Chem. Soc. 138, 2885–2888 (2016).
Fang, X., Yu, P. & Morandi, B. Catalytic reversible alkene-nitrile interconversion through controllable transfer hydrocyanation. Science 351, 832–836 (2016).
Rickborn, B. & Jensen, F. R. α-Carbon isomerization in amide dehydrations. J. Org. Chem. 27, 4608–4610 (1962).
Reisner, D. B. & Horning, E. C. Chloroacetonitrile. Org. Synth. 30, 22 (1950).
Krynitsky, J. A. & Carhart, H. W. Organic synthesis. Coll. 4, 436–438 (1963).
Narsaiah, A. V. & Nagaiah, K. An efficient and improved method for the preparation of nitriles from primary amides and aldoximes. Adv. Synth. Catal. 346, 1271–1274 (2004).
Maffioli, S. I., Marzorati, E. & Marazzi, A. Mild and reversible dehydration of primary amides with PdCl2 in aqueous acetonitrile. Org. Lett. 7, 5237–5239 (2005).
Zhang, W., Haskins, C. W., Yang, Y. & Dai, M. Synthesis of nitriles via palladium-catalyzed water shuffling from amides to acetonitrile. Org. Biomol. Chem. 12, 9109–9112 (2014).
Al-Huniti, M. H. et al. Development and utilization of a palladium-catalyzed dehydration of primary amides to form nitriles. Org. Lett. 20, 6046–6050 (2018).
Okabe, H., Naraoka, A., Isogawa, T., Oishi, S. & Naka, H. Acceptor-controlled transfer dehydration of amides to nitriles. Org. Lett. 21, 4767–4770 (2019).
Ishihara, K., Furuya, Y. & Yamamoto, H. Rhenium(VII) Oxo complexes as extremely active catalysts in the dehydration of primary amides and aldoximes to nitriles. Angew. Chem. Int. Ed. Engl. 41, 2983–2986 (2002).
Mineno, T. et al. Highly-efficient conversion of primary amides to nitriles using Indium(III) triflate as the catalyst. Int. J. Org. Chem. 4, 1–6 (2014).
Elangovan, S. et al. Knölker-type iron complexes bearing an N-heterocyclic carbene ligand: synthesis, characterization, and catalytic dehydration of primary Amides. Organometallics 34, 4521–4528 (2015).
Liu, R. Y., Bae, M. & Buchwald, S. L. Mechanistic insight facilitates discovery of a mild and efficient copper-catalyzed dehydration of primary amides to nitriles using hydrosilanes. J. Am. Chem. Soc. 140, 1627–1631 (2018).
Sykes, P. Chapter 4. A Guidebook to Mechanism in Organic Chemistry (6th edn) 77–100 (John Wiley & Sons, 1985).
Clayden, J., Greeves, N., Warren, S. & Wothers, P. Organic Chemistry 407–443 (Oxford University Press, 2001).
Smith, M. B. & March, J. March’s Advanced Organic Chemistry 6th edn 425–656 (John Wiley & Sons, 2007).
Villhauer, E. B. et al. 1-[[(3-hydroxy-1-adamantyl)amino]acetyl]-2-cyano-(S)-pyrrolidine: a potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties. J. Med. Chem. 46, 2774–2789 (2003).
Castaldi, M., Baratella, M., Menegotto, I. G., Castaldi, G. & Giovenzana, G. B. A concise and efficient synthesis of vildagliptin. Tetrahedron Lett. 58, 3426–3428 (2017).
Ahokoski, O. et al. Hormonal effects of MPV-2213ad, a new selective aromatase inhibitor, in healthy male subjects. A phase I study. Br. J. Clin. Pharmacol. 45, 141–146 (1998).
Ahokoski, O. et al. A double-blind study of MPV-2213ad, a novel aromatase inhibitor, in healthy male subjects. Eur. J. Clin. Pharmacol. 55, 27–34 (1999).
Ahokoski, O. et al. Pharmacokinetics of finrozole (MPV-2213ad), a novel selective aromatase inhibitor, in healthy men. Br. J. Clin. Pharm. 52, 702–704 (2001).
Sen, A., Dhital, R. N., Sato, T., Ohno, A. & Yamada, Y. M. A. Switching from biaryl formation to amidation with convoluted polymeric nickel catalysis. ACS Catal. 10, 14410–14418 (2020).
Noda, H., Furutachi, M., Asada, Y., Shibasaki, M. & Kumagai, N. Unique physicochemical and catalytic properties dictated by the B3NO2 ring system. Nat. Chem. 9, 571–577 (2017).
Hall, D. G. Boronic acid catalysis. Chem. Soc. Rev. 48, 3475–3496 (2019).
Sakakura, A., Ohkubo, T., Yamashita, R., Akakura, M. & Ishihara, K. Brønsted base-assisted boronic acid catalysis for the dehydrative intramolecular condensation of dicarboxylic acids. Org. Lett. 13, 892–895 (2011).
Zheng, H., Ghanbari, S., Nakamura, S. & Hall, D. G. Boronic acid catalysis as a mild and versatile strategy for direct carbo- and heterocyclizations of free allylic alcohols. Angew. Chem. Int. Ed. 51, 6187–6190 (2012).
Mo, X., Morgan, T. D. R., Ang, H. T. & Hall, D. G. Scope and mechanism of a true organocatalytic Beckmann rearrangement with a boronic acid/perfluoropinacol system under ambient conditions. J. Am. Chem. Soc. 140, 5264–5271 (2018).
Hashimoto, T., Galvez, A. O. & Maruoka, K. Boronic acid-catalyzed, highly enantioselective Aza-michael additions of hydroxamic acid to quinone imine ketals. J. Am. Chem. Soc. 137, 16016–16019 (2015).
Tanaka, M. et al. Boronic-acid-catalyzed regioselective and 1,2-cis-stereoselective glycosylation of unprotected sugar acceptors via SNi‑type mechanism. J. Am. Chem. Soc. 140, 3644–3651 (2018).
Leszczyński, P., Hofman, T. & Sporzyński, A. Solubility of phenylboronic acid and its cyclic esters in organic solvents. J. Solut. Chem. 49, 814–824 (2020).
Wang, Q., Tang, X.-T. & Shi, M. Metal-free cross-coupling of arylboronic acids and derivatives with DAST-type reagents for direct access to diverse aromatic sulfonamides and sulfonamide. Angew. Chem. Int. Ed. 55, 10811–10815 (2016).
Acknowledgements
We thank the materials characterization support team, CEMS, RIKEN for assistance with elemental analysis. We thank Prof. Zhaomin Hou (RIKEN Center for Sustainable Resource Science) for allowing us to use the mass spectrometer. We gratefully acknowledge financial support from AMED (grant no. 19ak0101115h), JSPS (Grant-in-Aid for Scientific Research (B) 21H01979; The Grant-in-Aid for Transformative Research Areas (A) JP21A204, Digitalization-driven Transformative Organic Synthesis (Digi-TOS)), Fugaku Trust for Medical Research, and RIKEN. HOKUSAI (RIKEN) provided the computer resources for the DFT calculations.
Author information
Authors and Affiliations
Contributions
A.S. and Y.M.A.Y. designed experiments. A.S. performed experiments. A.S. and Y.M.A.Y. wrote and revised the manuscript. A.M. performed calculations. A.O. performed compound characterization. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sen, A., Muranaka, A., Ohno, A. et al. Oxygen transfer reaction of haloalkyl amides catalyzed by phenylboronic acid. Commun Chem 6, 29 (2023). https://doi.org/10.1038/s42004-023-00824-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-023-00824-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.