Abstract
Ultrafast charge, energy and structural dynamics in molecules are driven by the topology of the multidimensional potential energy surfaces that determines the coordinated electronic and nuclear motion. These processes are also strongly influenced by the interaction with the molecular environment, making very challenging a general understanding of these dynamics on a microscopic level. Here we use electrospray and mass spectrometry technologies to produce isolated molecular ions with a controlled micro-environment. We measure ultrafast photo-induced ππ*-πσ* dynamics in tryptophan species in the presence of a single, charged adduct. A striking increase of the timescale by more than one order of magnitude is observed when changing the added adduct atom. A model is proposed to rationalize the results, based on the localized and delocalized effects of the adduct on the electronic structure of the molecule. These results offer perspectives to control ultrafast molecular processes by designing the micro-environment on the Angström length scale.
Similar content being viewed by others
Introduction
Ultrafast processes triggered by light absorption in biomolecules have prime importance in nature1,2,3. The complex interplay between electronic and nuclear degrees of freedom is responsible for information and energy transport, photoprotection, and structural changes, therefore essential to life sustainability. Understanding these processes at the microscopic atomic scale requires ultrafast pump–probe technology in order to determine the timescales as well as the degrees of freedom involved, and overall to decipher the multiple-step mechanisms induced by light4. To understand how nature has built these elementary processes, gas phase experiments represent a powerful tool as it allows to study the intrinsic properties of the molecule with a controlled (or absent) environment. Furthermore, experimental results can serve as a benchmark for high-level quantum chemistry calculations, eventually leading to a complete multiscale description of the link between electronic, geometrical structures, and reaction path.
Current ultrafast pump–probe experiments on gas-phase complex molecules are mostly performed on neutral compounds, demonstrating the prominence of this technique to unravel the subtle mechanisms at play in, e.g., ultrafast charge dynamics5, structural rearrangement6,7, and energy dissipation8,9,10, all involving non-adiabatic electro-nuclear dynamics. For biomolecules however, species are most often found in ionic forms (deprotonated, protonated, or complexed with a charged adduct) in biological conditions, where the charge state has a major influence on the chemical landscape and molecular reactivity. The development of ElectroSpray Ionization (ESI) technology has enabled the production of such charged molecules into the gas phase, opening new perspectives in the investigation of ionic species and in the development of relevant ultrafast experimental schemes11.
Ultrafast molecular processes are often driven by electronic or nuclear degrees of freedom as well as their couplings. In the Born–Oppenheimer representation, electronic states are defined along nuclear coordinates, and non-adiabatic dynamics occur at energy degeneracies where states cross12. A seminal example concerns the coupling between two states of different character, as the so-called ππ*–πσ* states, where the ππ* state corresponds to the excitation of typical aromatic chromophores, and the πσ* state has a σ character located on a specific X-H bond, with X being usually a heteroatom (N, O, or S). This prototypical situation is responsible for charge and energy transfer in aromatic based biologically relevant molecules13. For instance, experiments in aromatic species14,15, peptides containing aromatic amino acids16,17 or nucleotides18 have shown that photo-induced mechanisms driven by ππ*–πσ* couplings usually occur on very fast timescales.
Beyond the study of the natural processes occurring on ultrafast timescales, new possibilities to manipulate photo-induced reactions arise, offering new perspectives in chemistry or material science. Control strategies are numerous, spanning from coherent control that uses specially designed laser pulses to manipulate population transfer and couplings19, to specific bond excitation using infrared (IR) light in the particular case of charge transfer20, or chemical design where the atomic composition of the molecule is changed in order to improve a targeted photophysical property21.
Here we demonstrate that tailoring the micro-environment at the atomic scale in isolated amino acids and peptides allows us to tune the timescale of the ππ*–πσ* dynamics by more than one order of magnitude. We used a pump–probe scheme to measure this timescale variation in tryptophan-containing species as a function of a controlled micro-environment, comprising an adduct atom. Combined with quantum chemistry calculations, we show that this effect is due to the tuning of the relative energy between the ππ* and πσ* states, that is induced by the interaction with the adduct. This mechanism is deciphered by considering the separate effect of the adduct atom on the two states. It allows us to rationalize the behavior of other biomolecular structures as demonstrated in the case of a dipeptide, a first step towards more complex environments.
Results and discussion
Experiment
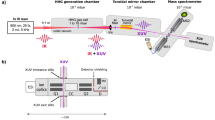
A schematic representation of the experimental setup is presented in Fig. 1a. It consists of a triple-quadrupole mass spectrometer instrument coupled to ultrafast laser technology22. In brief, molecular ions are produced in the gas phase using an ESI source. The ions are then filtered according to their mass-to-charge ratio by a first quadrupole (MS1). This allows the selection of the molecular ions of interest, which then interact with the laser pulses. The products of the interaction are analyzed by a second quadrupole mass filter (MS2). We used the output of a 25 fs, 800 nm, 2 mJ, 5 kHz laser to produce a pump ultraviolet (UV) pulse at 267 nm by third-harmonic generation. It is recombined with the 800 nm IR probe beam for time-resolved experiments. A typical pump–probe measurement consists of recording mass spectra obtained with MS2 at each UV–IR pump–probe delay. The dynamics is monitored by measuring the delay-dependent variation of the fragment signal. To do so the signal obtained following the interaction with the UV pump only is subtracted from the signal obtained with the pump and the probe, for each delay. Compared to other existing configurations using an ion trap, the perpendicular, “on-the-fly”, design ensures that molecular ions interact only once with the pump and probe pulses, therefore simplifying the interpretation.
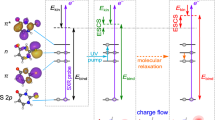
a Experimental setup for time-resolved spectroscopy of on-the-fly molecular ions, combining an electrospray source (ESI), mass selection (MS1), laser interaction, and detection (MS2). b Concept of Global-Local Stabilization Separation (GLoSS), illustrated in the case of tryptophan-containing species: after absorption (in the UV range in this work), the relative energy gap εg between the excited ππ* state (in blue, the red shading indicates the excited electron density) and the πσ* state (in red, the blue shading indicates the excited electron density) can be tuned to directly change the ππ*–πσ* transfer timescale. More precisely, the two states can be changed in energy by playing separately on the complexation interaction of the molecule with the adduct atom (global stabilization acting on the ground state) or on the specific interaction of the adduct with the NH2 moiety (local stabilization), thus changing their energy gap (in this figure, the πσ* state is represented as a bond state for sake of simplicity, while it corresponds to a dissociative state).
The mass spectrum obtained following photoexcitation of sodiated tryptophan (TrpNa+, m/z = 227) at 267 nm is presented in Fig. 2a. It shows that laser interaction with the ions dominantly leads to photofragment m/z = 130, which is barely present in statistical dissociation experiments such as collision-induced dissociation (CID)23,24. This UV-induced fragment corresponds to Cα–Cβ cleavage as indicated in Fig. 2a25, and shows that specific dynamics are at play after absorption to the first excited states.
a Mass spectrum of sodiated tryptophan (m/z = 227, indicated by the † marker), obtained after UV irradiation. The * markers depict residual (CID) fragments, and the structure of fragment m/z = 130 (Cα–Cβ cleavage in purple) is shown. Intensity on the y-axis represents the signal normalized on the parent intensity (i.e., m/z = 227 is 100%). b Normalized time-dependent yield of fragment m/z = 130 in TrpH+, as a function of the UV pump–IR probe delay (blue dots), together with its exponential fit (dark green line), yielding a timescale of 390 ± 100 fs. c, d Same analysis for TrpNa+ (orange dots) and AlaTrpNa+ (red dots), giving timescales of 13 ± 3 ps (purple line) and 35 ± 8 ps (dark red line) respectively. The chemical structure of the three molecules is shown in each box. In the case of AlaTrpNa+, fragment m/z = 130 is not observed, and the dynamics is observed in, e.g., fragment m/z = 168, as shown in (d) and in Supplementary Fig. 3.
In order to measure the dynamics induced by UV absorption, we performed UV–IR pump–probe measurements with our on-the-fly apparatus. We recorded the variation of the m/z = 130 photofragment yield as a function of the pump–probe delay, as shown in Fig. 2c. A clear time-dependent signal is obtained showing a very fast rise of the signal around the UV–IR temporal overlap followed by an exponential decay in the picosecond time domain, which eventually leads to a steady contribution (step). In the experiment, the pump pulse excites the parent ππ* state, and the probe pulse promotes the population of the excited state to higher lying states, eventually leading to fragmentation. The delay-dependent signal of the m/z 130 photofragment is thus a signature of the dynamics in the ππ* state. Let us notice that this fragment is simply a convenient signature of the dynamics occurring in the parent ππ* excited state, as such that the extracted timescale is related to the excited state dynamics and not to the fragmentation process itself.
Fitting the data using a single exponential model yielded τ = 13 ± 3 ps. Following the same procedure, we studied the dynamics in two other tryptophan-containing systems: protonated tryptophan (TrpH+) and sodiated alanyl-tryptophan (AlaTrpNa+). These two molecules are built on the same chromophore as TrpNa+ (the indole ring) but with modifications in the ion adduct or the peptide chain. The results of the time-dependent measurements are presented in Fig. 2b (TrpH+) and Fig. 2d (AlaTrpNa+). In the case of TrpH+, the same fitting procedure yielded a timescale of 390 ± 100 fs, in very good agreement with results reported in the literature (380 ± 50 fs)26,27,28. For AlaTrpNa+, the extracted timescale is 35 ± 8 ps. In all cases, a similar behavior is obtained, corresponding to a fast dynamics driven by a single decay (see Section 1 of Supplementary Methods and Supplementary Figs. 1–3 for more details). However, the extracted timescale becomes slower and slower when changing from TrpH+ to TrpNa+ (the decay time increases by a factor 30) and to AlaTrpNa+ (increase by a factor 100), the timescale being thus tuned on two orders of magnitude. We note that this striking variation is obtained by simply changing the local environment of the indole chromophore, without affecting the molecular backbone.
Calculations
In order to rationalize the observed trend, excited-state calculations were performed on the three molecular compounds. Indeed, UV absorption of indole-based molecules is governed by ππ* transitions, where the excited electron density is located on the indole ring. Following geometry optimization using density functional theory (DFT) at the B3LYP/6-311+G(d,p) level of theory, RICC2/aug-cc-pVDZ calculations were conducted for the energy of the electronic excited states, as well as calculations of their related potential energy surfaces (PESs). The result for the excited states is presented in Fig. 3, together with the ground state geometries. ππ* states are identified as the blue states, whose oscillator strength is the strongest. Additionally, two different states are present, with a πσ* and ππCO* electronic character, respectively. In the three molecules, the πσ* state is localized on the NH2X moiety, and lies in the vicinity of the excited ππ* states. One can thus expect ultrafast dynamics to occur due to non-adiabatic couplings between these two states. To confirm the presence of such couplings, we computed the PESs for TrpNa+. We studied the reaction along the N–X bonds, as they are known as a major pathway in ππ*–πσ* dynamics14. PESs reported in Fig. 4 show that a non-adiabatic crossing occurs between the ππ* and πσ* states: while the πσ* state is bound along the N–H coordinate (Fig. 4a), it is dissociative along the N-Na coordinate, crossing the ππ* state (Fig. 4b). Moreover, the dissociative character of the πσ* state is responsible for the specific fragmentation pattern observed in the experiment, on longer timescales.
Calculated vertical excited states for TrpH+ (a), TrpNa+ (b), and AlaTrpNa+ (c), together with their equilibrium geometry (on top). The corresponding dominant orbitals are also displayed, giving the state-character of the excited state (πσ* states in red, ππ* states in blue, ππCO* state in green, with the ground state being in dark). Changing the adduct atom (from a) to b)) or changing the molecular backbone (from b) to c)) has a different influence on the position of the πσ* states and ππ* states.
Computed potential energy surfaces for TrpNa+, along the N–H (a) and N–Na (b) stretching modes, depicted by the geometries on top. The ground state equilibrium geometry corresponds to RNH = 1.02 Å and RNNa = 2.47 Å. The πσ* state is displayed in red, ππ* states are displayed in blue, and the ground state is displayed in orange.
The described mechanism is very similar for the two other species (see section 4 of Supplementary Methods and Supplementary Figs. 6, 7 for other PESs). Thus, as shown schematically in Fig. 1b, the observed dynamics corresponds to the ππ*–πσ* non-adiabatic coupling for the three molecules, enabling a population transfer to the πσ* state following local excitation of the indole ring. Within this framework, the transfer timescale τ is expected to strongly depend on the energy gap εg between the two states according to simple energy gap considerations. This is indeed observed in our calculations (Fig. 3): when changing from TrpH+ to AlaTrpNa+, little change occurs in the position of the ππ* states while a drastic increase in the energy of the πσ* state is noticed (from 4.77–5.16 eV). Consequently, the striking increase of the timescale is a direct consequence of the increase in the energy gap between the coupled states, due to changes in the micro-environment, as observed for substituted neutral heteroatomic molecules29,30,31,32 or water-ion complexes33. Let us note that the reported energies are vertical energies from the ground state minimum energy geometry. Although the adiabatic energies usually serve as a reference when considering non-adiabatic dynamics, the choice of vertical energies allows us to have the same reference for all molecules even in the case of dissociative states or more complex PES.
To go deeper in the understanding of this effect, it is instructive to understand the key parameters imposed by the environment. For a well-defined molecular system (tryptophan-based ions in this study), the effect of the adduct atom can be understood in terms of energetic stabilization of the electronic states involved. Here, the excited ππ* state corresponds to an electron density localized on the UV chromophore, i.e., the indole ring, as in the ground state. This means that both the ground and ππ* states will be sensitive to the global interaction of the molecule with the adduct atom. In contrast, the πσ* state corresponds to an electron density localized on the N-terminus, i.e., its localization differs from the ground and ππ* states. It is therefore sensitive to the local interaction with the adduct atom, because the electron density in this state becomes sensitive to the electrostatic interaction in the region of the NH2X moiety.
In this picture, the increase of the energy gap observed in the results of the RICC2/aug-cc-pVDZ calculations can be rationalized by global and local stabilization of the ππ* and πσ* states when changing the adduct atom. The energy change of the ππ* state induced by the global effect of the adduct depends mainly on the global affinity of Trp for the considered adduct X+, called XA thereafter. On the other hand, the energy change of the πσ* state due to local effects of the adduct is mainly determined by interactions between the NH2 moiety and the adduct X+ (Fig. 1b). In a first approximation, this interaction is considered as a long-range Coulomb attraction (ECoul, see Supplementary Discussion for more details). These simple considerations lead to the following expression for the energy gap increase:
where εg = Eπσ*−Eππ* is the (relative) energy gap between the πσ* state and the ππ* state for a given species, associated to a specific chromophore (here the indole ring). δεg is the variation of the energy gap when changing from one adduct to another, e.g., from H to Na. ΔXA and ΔECoul are respectively the changes in affinity for the adduct atom X and in Coulomb energy when changing the adduct. Finally, RNX is the equilibrium distance between the adduct X and the NH2 moiety. For the specific case of TrpH+ and TrpNa+, considering the different affinities34 and equilibrium distances for the proton and sodium adducts, this leads to an increase of the energy gap of δεg = 395 meV from TrpH+ to TrpNa+, which is strikingly close to the result of the ab initio calculations, that predict an increase of δεg = 310 meV.
Based on this simple model it becomes straightforward to understand the process in terms of the combined global and local effects of the adduct atom on the molecule. This Global-Local Stabilization Separation model (GLoSS) is very informative since it enables to disentangle each contribution and how it will influence the timescale of the dynamics: increasing the global adduct affinity will increase the timescale by increasing the energy gap, while a closer NH2–X distance decreases the timescale by increasing the Coulomb stabilization of the πσ* state.
The GLoSS model can be further illustrated in the case of AlaTrpNa+. In this case, the adduct atom remains unchanged (Na+) compared to TrpNa+, but the environment of the indole chromophore becomes more flexible because of the alanyl residue. Thus, the global complexation of the Na+ adduct is more favorable than for TrpNa+, resulting in an increase of the sodium affinity35, and based on the GLoSS model it should increase further the ππ*–πσ* timescale, which is indeed observed (Fig. 2). This increase arises from the separated stabilization of the ππ* state by complexation, while the πσ* state is unaffected because the NH2-Na distance remains constant between TrpNa+ and AlaTrpNa+. Again, the GLoSS model gives a good agreement with ab initio calculations (δεg = 326 meV when AlaTrpNa+ is compared to TrpNa+, instead of 230 meV in the calculations), showing a clear illustration of the separation of the stabilization contributions.
While this ππ*–πσ* dynamics is associated with non-adiabatic couplings between the two states, it can also be seen as a charge transfer (CT) process. Because of the difference in the density localization of the states, the dynamics implies an electron transfer from one side of the molecule to another, as can be clearly seen in Fig. 3. Such a CT process was also invoked in the relaxation of protonated tryptophan26,27. In this scenario, the species absorb UV light through the ππ* excitation localized on the indole chromophore and it has been shown that a CT process might occur through the ππ*-πσ* coupling described in this work. The initial positive charge, borne by the NH3+ terminus, transits to the indole moiety when the population is transferred to the πσ* state36,37. In the case of sodiated species studied here, the same process occurs, showing that CT dynamics can be controlled using the micro-environment.
In conclusion, we have shown that ππ*–πσ* dynamics can be controlled by using the influence of an adduct atom positioned on the Angström length scale. On a quantum mechanical point of view, the three molecules investigated have similar electronic structures, which ensures that the mechanism can be described by a limited number of involved states. The influence of the adduct is rationalized as a tuning of the relative energy gap between the ππ* and the πσ* states that strongly influences the non-adiabatic coupling efficiency, and thus the femtosecond-to-picosecond timescale. This mechanism is general and can be understood as the mixed contribution of the role of global and local stabilization effects. These contributions can be disentangled in a GLoSS model based on first principle arguments. Such a model, where the different stabilization contributions are separated, could be further used to molecular systems with states of different electronic characters, as performed here for ππ*–πσ* dynamics. In turn, it offers simple control strategies based on atomic length scale designed micro-environment of more or less flexible molecular structures to influence the charge and energy flow. Although this work focuses on the influence of adduct atoms on ππ*–πσ* dynamics in peptides, the UV-induced dynamics in these species is rather complex and many other aspects might be investigated in the future. More generally, other processes such as structural changes, including isomerization, or spin effects (role of triplet states) might also be considered in the perspective of a controlled micro-environment, calling for new experimental investigations.
Methods section
Experimental section
Pump–probe measurements were conducted using a UV–IR interferometer. The laser output (800 nm, 25 fs, 2 mJ, 5 kHz) was first used to generate 267 nm photons by third-harmonic generation (using two BBO crystals of 200 µm and 50 µm thicknesses). The UV pump beam is then separated from the residual IR light by a dichroic beamsplitter. It passes through a reflective delay line, before being recombined with the IR probe beam using a second dichroic beamsplitter. In this experiment, the IR probe beam consists of the residual IR that was not converted into UV photons. Its energy is controlled using a half-wave plate and a polarizer, and a second half-wave plate is used to control the polarization. Both beams are focused collinearly using an f = 1 m lens on the ion beam, which is produced by the triple-quadrupole instrument. The IR probe focus was kept 10 cm away from the ion beam, to fully overlap the UV beam spot and avoid extensive ionization. The UV and IR energies were 5 µJ and 800 µJ, respectively, yielding an IR intensity of 1–2 × 1012 W cm−2. Due to this low intensity, no ionization nor fragmentation induced by the IR pulse alone was detected, and the probe mechanism thus consists of perturbative absorption to higher lying excited states of the molecules. For each pump–probe delay, an average mass spectrum was recorded over 40 s.
After integration of the yield of the different photofragments for each delay, a fitting procedure was used, using the following formula:
where τ is the lifetime of the exponential decay, t0 the delay at which the pump and probe pulses overlap temporally, Astep the amplitude difference between the yield at infinite negative and positive delays, Adecay the amplitude of the decay, and θ the Heaviside function. For AlaTrpNa+, the same (τ,t0) parameters were fitted for all the fragments in a multidimensional procedure. In the case of TrpH+, the signal was fitted by the convolution of Eq. (2) and a gaussian cross-correlation, due to finite pulse durations. Error bars of the extracted lifetime correspond to standard deviation of the least-square fitting procedure.
Chemicals were purchased from Sigma-Aldrich, and solutions were prepared at a concentration of 200 µM in a 50:50 mixture of MeOH:H2O. 0.1% of acetic acid was added to the solution for the production of TrpH+, and NaCl was added to a concentration of 0.1 mM for the production of TrpNa+ and AlaTrpNa+.
Computational methods
Geometries of the ground states of TrpH+, TrpNa+, and AlaTrpNa+ were optimized by DFT calculations using the B3LYP functional at the 6-311+G(d,p) level (see Supplementary Tables 6–8 for the atomic coordinates). For the most stable conformer of each species, the excited states have been calculated using RICC2 computations, at the aug-cc-pVDZ level, known to give good results in these protonated systems11,37,38. As mentioned in Sections 2–3 of the Supplementary Methods, we also conducted these calculations for higher-energy conformers (Supplementary Figs. 4–5 and Supplementary Tables 1, 3) as well as for different basis sets and geometries (Supplementary Table 2 and Supplementary Fig. 8), where the overall energetic position of the states remains similar to the most stable conformer. Finally, the potential energy surfaces were constructed by calculating the excited states while stretching the considered bond, without further geometry optimization. Further details about the geometries, excited states and PESs can be found in Sections 2–4 of the Supplementary Methods. Details about the results and derivation of the GLoSS model are given in Supplementary Discussion, Supplementary Figs. 9–10 and Supplementary Tables 4, 5.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Zewail, A. H. Femtochemistry: atomic-scale dynamics of the chemical bond using ultrafast lasers (nobel lecture). Angew. Chem. Int. Ed. 39, 2586–2631 (2000).
Bixon, M. & Jortner, J. Electron transfer-from isolated molecules to biomolecules. Adv. Chem. Phys. 106, 35–201 (1999).
May, V. & Kühn, O. Charge and Energy Transfer Dynamics in Molecular Systems (John Wiley & Sons, 2011).
Stolow, A., Bragg, A. E. & Neumark, D. N. Femtosecond time-resolved photoelectron spectroscopy. Chem. Rev. 104, 1719–1758 (2004).
Neville, S. P., Kirkby, O. M., Kaltsoyannis, N., Worth, A. G. & Fielding, H. H. Identification of a new electron-transfer relaxation pathway in photoexcited pyrrole dimers. Nat. Commun. 7, 11357 (2016).
Pathak, S. et al. Tracking the ultraviolet-induced photochemistry of thiophenone during and after ultrafast ring opening. Nat. Chem. 12, 795–800 (2020).
Attar, A. R. et al. Femtosecond x-ray spectroscopy of an electrocyclic ring-opening reaction. Science 356, 54–59 (2017).
Wolf, T. J. A. et al. Probing ultrafast ππ*/nπ* internal conversion in organic chromophores via K-edge resonant absorption. Nat. Commun. 8, 29 (2017).
Squibb, R. J. et al. Acetylacetone photodynamics at a seeded free-electron laser. Nat. Commun. 9, 63 (2018).
Corrales, M. E., González-Vázquez, J., de Nalda, R. & Bañares, L. Coulomb explosion imaging for the visualization of a conical intersection. J. Phys. Chem. Lett. 10, 138–143 (2019).
Soorkia, S., Jouvet, C. & Grégoire, G. UV photoinduced dynamics of conformer-resolved aromatic peptides. Chem. Rev. 120, 3296–3327 (2020).
Domcke, W. & Yarkony, D. R. Role of conical intersections in molecular spectroscopy and photoinduced chemical dynamics. Annu. Rev. Phys. Chem. 63, 325–352 (2012).
Sobolewski, A. L. G., Domcke, W., Dedonder-Lardeux, C. & Jouvet, C. Excited-state hydrogen detachment and hydrogen transfer driven by repulsive 1πσ* States: a new paradigm for nonradiative decay in aromatic biomolecules. Phys. Chem. Chem. Phys. 4, 1093–1100 (2002).
Ashfold, M. N. R., Cronin, B., Devine, A. L., Dixon, R. N. & Nix, M. G. D. The role of πσ* excited states in the photodissociation of heteroaromatic molecules. Science 312, 1637–1640 (2006).
Ashfold, M. N. R. et al. Exploring nuclear motion through conical intersections in the UV photodissociation of phenols and thiophenol. Proc. Natl Acad. Sci. USA 105, 12701–12706 (2008).
Nolting, D., Schultz, T., Hertel, I. V. & Weinkauf, R. Excited state dynamics and fragmentation channels of the protonated dipeptide H2N-Leu-Trp-COOH. Phys. Chem. Chem. Phys. 8, 5247–5254 (2006).
Grégoire, G. et al. Statistical vs. non-statistical deactivation pathways in the UV photo-fragmentation of protonated tryptophan–leucine dipeptide. Phys. Chem. Chem. Phys. 8, 122–128 (2006).
Satzger, H. et al. Primary processes underlying the photostability of isolated DNA bases: adenine. Proc. Natl Acad. Sci. USA 103, 10196–10201 (2006).
Nuernberger, P., Vogt, G., Brixner, T. & Gerber, G. Femtosecond quantum control of molecular dynamics in the condensed phase. Phys. Chem. Chem. Phys. 9, 2470–2497 (2007).
Delor, M., Sazanovich, I. V., Towrie, M. & Weinstein, J. A. Probing and exploiting the interplay between nuclear and electronic motion in charge transfer processes. Acc. Chem. Res. 48, 1131–1139 (2015).
Gueye, M. et al. Engineering the vibrational coherence of vision into a synthetic molecular device. Nat. Commun. 9, 313 (2018).
Hervé, M. et al. On-the-fly femtosecond action spectroscopy of charged cyanine dyes: electronic structure versus geometry. J. Phys. Chem. Lett. 10, 2300–2305 (2019).
El Aribi, H., Orlova, G., Hopkinson, A. C. ; & Michael Siu, K. W. Gas-phase fragmentation reactions of protonated aromatic amino acids: concomitant and consecutive neutral eliminations and radical cation formations. J. Phys. Chem. A 108, 3844–3853 (2004).
Lioe, H., O’Hair, R. A. J. & Reid, G. E. Gas-phase reactions of protonated tryptophan. J. Am. Soc. Mass Spectrom. 15, 65–76 (2004).
Kang, H. et al. Photo-induced dissociation of protonated tryptophan TrpH+: a direct dissociation channel in the excited states controls the hydrogen atom loss. Phys. Chem. Chem. Phys. 6, 2628–2632 (2004).
Kang, H. et al. Ultrafast deactivation mechanisms of protonated aromatic amino acids following UV excitation. Phys. Chem. Chem. Phys. 7, 394–398 (2005).
Kang, H. et al. Control of bond-cleaving reactions of free protonated tryptophan ion by femtosecond laser pulses. J. Phys. Chem. A 109, 2417–2420 (2005).
Boyarkin, O. V., Mercier, S. R., Kamariotis, A. & Rizzo, T. R. Electronic spectroscopy of cold, protonated tryptophan and tyrosine. J. Am. Chem. Soc. 128, 2816–2817 (2006).
Pino, G. A. et al. Excited state hydrogen transfer dynamics in substituted phenols and their complexes with ammonia: ππ*-πσ* energy gap propensity and ortho-substitution effect. J. Chem. Phys. 133, 124313 (2010).
Karsili, T. N. V. et al. O–H bond fission in 4-substituted phenols: S1 state predissociation viewed in a Hammett-like framework. Chem. Sci. 4, 2434–2446 (2013).
Wenge, A. M. et al. Tuning photochemistry: substituent effects on πσ* state mediated bond fission in thioanisoles. Phys. Chem. Chem. Phys. 17, 16246–16256 (2015).
Kinoshita, S. et al. Substitution effect on the nonradiative decay and trans → cis photoisomerization route: a guideline to develop efficient cinnamate-based sunscreens. Phys. Chem. Chem. Phys. 23, 834–845 (2021).
Mercier, S. R. et al. Microsolvation effects on the excited-state dynamics of protonated tryptophan. J. Am. Chem. Soc. 128, 16938–16943 (2006).
Kish, M. M., Ohanession, G. & Wesdemiotis, C. The Na+ affinities of α-amino acids: side-chain substituent effects. Int. J. Mass Spectrom. 227, 509–524 (2003).
Wang, P., Wesdemiotis, C., Kapota, C. & Ohanessian, G. The sodium ion affinities of simple di-, Tri-, and tetrapeptides. J. Am. Soc. Mass Spectrom. 18, 541–552 (2007).
Grégoire, G., Jouvet, C., Dedonder, C. & Sobolewski, A. L. Ab initio study of the excited-state deactivation pathways of protonated tryptophan and tyrosine. J. Am. Chem. Soc. 129, 6223–6231 (2007).
Kadhane, U. et al. Photodissociation of protonated tryptophan and alteration of dissociation pathways by complexation with crown ether. J. Phys. Chem. 129, 184304 (2008).
Grégoire, G. et al. UV photoinduced dynamics in protonated aromatic amino acid. Eur. Phys. J. D. 51, 109–116 (2009).
Acknowledgements
The authors thank Gilles Grégoire and Satchin Soorkia for fruitful discussions concerning the experimental results, the interpretation and for providing NPA calculations. The authors thank Baptiste Schindler, Vincent Loriot, and Eric Constant for their help on the technical implementation. The research has been supported by CNRS, ANR-16-CE30-0012 “Circé”. In this work, we were granted access to the HPC resources of the FLMSN, “Fédération Lyonnaise de Modélisation et Sciences Numériques”, partner of EQUIPEX EQUIP@MESO and to the “Centre de calcul CC-IN2P3” at Villeurbanne, France.
Author information
Authors and Affiliations
Contributions
M.H., I.C. and F.L. conceived the project. M.H., A.B., R.B., I.C. and F.L. conducted the experiments. M.H. and A.B. performed the data analysis. A.A. performed the calculations and analyzed it together with M.H.A.A. and F.L. developed the interpretation model and wrote the manuscript, with inputs from all the authors. I.C. and F.L. led the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hervé, M., Boyer, A., Brédy, R. et al. Controlled ultrafast ππ*-πσ* dynamics in tryptophan-based peptides with tailored micro-environment. Commun Chem 4, 124 (2021). https://doi.org/10.1038/s42004-021-00557-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-021-00557-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.