Abstract
The telencephalon has undergone remarkable diversification and expansion throughout vertebrate evolution, exhibiting striking variations in structural and functional complexity. Nevertheless, fundamental features are shared across vertebrate taxa, such as the presence of distinct regions including the pallium, subpallium, and olfactory structures. Teleost fishes have a uniquely “everted” telencephalon, which has confounded comparisons of their brain regions to other vertebrates. Here we combine spatial transcriptomics and single nucleus RNA-sequencing to generate a spatially-resolved transcriptional atlas of the Mchenga conophorus cichlid fish telencephalon. We then compare cell-types and anatomical regions in the cichlid telencephalon with those in amphibians, reptiles, birds, and mammals. We uncover striking transcriptional similarities between cell-types in the fish telencephalon and subpallial, hippocampal, and cortical cell-types in tetrapods, and find support for partial eversion of the teleost telencephalon. Ultimately, our work lends new insights into the organization and evolution of conserved cell-types and regions in the vertebrate forebrain.
Similar content being viewed by others
Introduction
The forebrain houses regions that regulate complex functions such as learning, memory, and social behavior. Despite its conserved functions, this structure has grown larger and increasingly complex over evolutionary time, exhibiting marked variations across vertebrate taxa including the mammalian six-layered neocortex, the dorsal ventricular ridge (DVR) in sauropsids (reptiles and birds), and the “everted” teleost pallium of ray-finned fish (Actinopterygii). As a result, the evolutionary relationships of specific forebrain regions across species are still actively debated.
Several subregions of the teleost telencephalon have been compared to mammalian brain regions involved in regulating social behaviors, including the hippocampus, striatum, and septum1. However, establishing homologues between telencephalic subdivisions in fish to those of distantly-related vertebrate clades has been historically challenging, due in part to the “everted” morphology of the teleost telencephalon. The unique outward folding of the teleost pallium during development, known as eversion, leads to an altered arrangement of pallial zones compared to the “evaginated” brains of other vertebrate lineages2,3. Multiple proposed models of eversion attempt to identify the counterparts of other vertebrate pallial territories in fish2,3,4,5,6. However, these models are unresolved, leaving the evolutionary identities of most divisions of the teleost pallium in question. Modern single cell and spatial omics technologies are powerful tools to address these long-standing questions. Recent studies in different tetrapod lineages have begun to utilize these techniques to investigate vertebrate brain evolution at an unprecedented resolution7,8,9,10,11,12. Advances in methods for comparative analysis of these data have further increased the ability to establish homologous cell-types across distant phyla13,14.
We recently employed single nucleus RNA-sequencing (snRNA-seq) to link genomic signatures of behavioral evolution to specific cell populations in the cichlid telencephalon15. Here, we perform spatial transcriptomics (ST) and map cell populations identified by snRNA-seq to create a spatially resolved transcriptional atlas of the cichlid telencephalon. We then survey the cellular architecture of this brain structure and compare its component cell-types and brain regions across all five major vertebrate lineages. Our work generates new and unanticipated insights into teleost neurodevelopment and vertebrate forebrain evolution.
Results
Spatially resolved gene expression profiles in the teleost brain
To investigate the anatomical landscape of the adult Mchenga conophoros (MC) telencephalon, we generated ST data using the 10x Genomics Visium platform (Fig. 1a). We dissected the telencephala from two adult male MCs (Supplementary Data 1), collecting four representative 10 μm coronal sections along the rostrocaudal axis per subject on the individual capture areas of 10x Visium slides (Fig. 1b; Fig. S1). Seven total sections (n = 3 from subject 1, n = 4 from subject 2) were chosen for downstream analysis based on tissue quality (Fig. S1). Due to rostrocaudal variation between the left and right telencephalic hemispheres on individual capture areas, we visualized and analyzed each hemisphere separately (Fig. 1b). In total, >500 million RNA reads (68 ± 2 million reads per capture area) were sequenced and aligned to the cichlid Maylandia zebra reference genome16. A total of 3971 spots (567 ± 65 spots per capture area) contained tissue and at least one unique molecular identifier (UMI), averaging 9729 ± 81 UMIs (range: 73–37,124) and 3639 ± 20 genes per spot (range: 73–7263; Fig. S2). Clustering parameters were chosen systematically using ChooseR, which evaluates clustering quality based on robustness metrics of bootstraps (Fig. S3; Supplementary Data 2). Using these near optimal parameters, 33 clusters of spots were identified (Fig. 1c, d). Marker genes of clusters were determined (Supplementary Data 3) and among these were canonical markers of well-defined anatomical regions in the teleost telencephalon (Fig. S4, Supplementary Note 1).
a Overview of spatial transcriptomics experimental pipeline. 10 μm coronal sections from cryopreserved MC telencephala were placed on capture areas of 10x Genomics Visium slides and subsequently fixed, H&E stained, and imaged. Tissue was permeabilized for mRNA capture by barcoded primers on 50μm-diameter spots and spatially-barcoded cDNA was processed for downstream library preparation and sequencing. b Schematic of experimental design including four tissue sections on capture areas (C1-4) from two male MC subjects (subject 1, S1; subject 2, S2), totaling eight samples processed on the 10x Visium platform. Tissue from the left and right telencephalic hemispheres (L and R) were visualized separately. c Unsupervised gene expression clustering of spots visualized in UMAP space. D. 50 μm-diameter spots on select tissue section hemispheres are colored by clusters from (d) The order of tissue sections along the rostrocaudal axis was determined through visual inspection of H&E-stained tissue.
Clusters correspond with anatomical regions
Anatomical regions were manually annotated based on tissue cytoarchitecture and expression of select canonical gene markers (Fig. 2a; Supplementary Note 1) and the distribution of clusters across these regions was assessed (Fig. S5). We observed high correspondence between anatomical regions and clusters (Rand index = 0.9487354), suggesting that transcriptional profiles of spots are strongly associated with their respective brain regions of origin.
a Anatomical annotation of select tissue sections (order same as Fig. 1) with representative coronal atlases shown on the left and spots colored by anatomical region on the right. Dc=central division of D, Dc-1,2,3,4,5=subdivisions of Dc, Dd=dorsal division of D, Dl=lateral division of D, Dl-d=dorsal subdivision of Dl, Dl-g=granular zone of Dl, Dl-v=ventral subdivision of Dl, Dl-vv=ventral zone of Dl-v, Dm=medial division of D, Dm-1,2,3=subdivisions of Dm, Dm-2c=caudal part of Dm-2, Dm-2r=rostral part of Dm-2, Dp=posterior division of D, OB gc=olfactory bulb granule (internal) cell layer, OB gml=olfactory bulb glomerular and mitral (external) cell layers, Vc=central nucleus of V, Vd=dorsal nucleus of V, Vd-c=caudal part of Vd, Vd-r=rostral part of Vd, Vi=intermediate nucleus of V, Vl=lateral nucleus of V, Vs=supracommissural nucleus of V, Vv=ventral nucleus of V, Vx=unassigned subdivision of V, VZ=ventricular zone, vVZ=ventral ventricular zone, ON=olfactory nerve. b Spots visualized in UMAP space with anatomical annotations. Also shown is the expression of ventral (dlx5), dorsal (bhlhe22), excitatory (slc17a6), and inhibitory (gad1) marker genes. c Expression of neuromodulatory genes in representative section hemispheres, with transparency of spots scaled by expression level (spots not expressing the gene are completely transparent).
The teleost telencephalon is subdivided into pallial (dorsal, D) and subpallial (ventral, V) domains which are predominantly populated by glutamatergic or GABAergic neurons, respectively. We identified these divisions in ST data based on expression patterns of conserved pallial (bhlhe22, neurod1, neurod6b, eomesa) and subpallial (dlx2, dlx5) marker genes (Fig. 2b; Fig. S6; Supplementary Note 1)12,17,18. As expected, we observed strong glutamatergic marker gene expression in pallial regions (slc17a6, slc17a7a) and GABAergic expression dominating subpallial regions (gad1, gad2; Fig. 2b; Fig. S6; Supplementary Note 1)19.
Gene expression properties of profiled anatomical regions aligned with patterns described in previous teleost studies, briefly summarized below. Subpallial regions Vv (ventral nucleus of V) and Vl (lateral nucleus of V) are considered putatively homologous to the septal formation in mammals1. In these regions, we observed expression of markers in agreement with previous reports in fish (Vv: isl1a, lhx6a, lhx8, and zic117,20; Vl: sst1.1, npy and crhb1,21,22,23; Fig. 2c, Fig. S6). The olfactory bulb granule cell layer (OB gc) expressed dopaminergic cell-type marker tyrosine hydroxylase (th)24,25 along with pax6, a gene necessary for adult neurogenesis of dopaminergic neurons in the mammalian OB (Fig. 2c; Figs. S6–7)26. The dorsal, supracommissural, and central nuclei of V (Vd, Vs, and Vc, respectively) expressed markers of mammalian striatal medium spiny neurons (MSNs; penka, meis2, six3a; Fig. 2c, Fig. S6)27,28 and genes involved in dopaminergic transmission (slc18a2, slc6a3; Fig. S6)24, in agreement with previously reported similarities between these regions and the mammalian striatum1,29. Teleost pallial regions Dl (lateral division of D) and Dm (medial division of D) are considered the putative homologues of the mammalian medial and ventral pallium, respectively30. In zebrafish, parvalbumin expression in the pallium has been used to establish histogenetic units, as it is observed in the Dl but not the Dm31. Interestingly, this pattern is not replicated in our data, as both Dl and Dm expressed pvalb7 (Fig. 2c; Fig. S6), though this result may be due to differences in RNA versus protein expression. Urocortin (ucn/uts1) was highly expressed throughout the pallium, consistent with previous observations in teleosts (Fig. 2c; Fig. S6)22,32.
The majority of teleost gene expression studies have been limited by the necessary identification of gene targets prior to experimentation. The advent of ST technology eliminates this necessity, allowing anatomically-resolved profiling of thousands of genes simultaneously. In addition to replicating previously reported gene expression patterns, ST allowed us to identify novel marker genes for specific brain regions (Supplementary Data 4, Fig. S6).
Anatomical distribution of cell-types in the telencephalon
The 10x Visium platform is a powerful tool for investigating the expression of thousands of genes within tissue anatomy, but lacks precise cellular resolution as each 50 μm-diameter spot may capture RNA from multiple cells and cell-types. Algorithms for cell-type deconvolution of spots address this limitation by estimating the cellular composition of individual spots. We recently profiled the cell-types in the MC telencephalon (38 subjects and 33,674 nuclei) using snRNA-seq15. Here, we predicted the anatomical location of these cell-types in our ST data using cell2location33 (Fig. 3a, b), a top performing tool for cell-type deconvolution of spatial spots34. Spots were predicted to contain on average 8.7 ± 0.086 cells (Fig. S8; Supplementary Data 5, Supplementary Note 2) and most (>92%) were composed of more than one cell-type (4.3 ± 0.032 cell-types per spot on average). In general, anatomical locations of snRNA-seq cell-types (Fig. 3a, b) agreed with predictions based on previously-reported gene expression patterns in teleosts. For example, 5_GABA cell-types, which demonstrated expression of well-known teleost OB marker genes (e.g., th, pax6)24,25,26, mapped to the OB (Fig. 3c) and 4_GABA cell-types which expressed genes enriched in striatal MSNs (sp9, six3a)35 were putatively located in the Vd and Vs regions (Fig. 3c). Our ST data not only represents a valuable resource for investigating the anatomical distributions of genes and cell-types in MC and other closely related cichlid species, but it can additionally serve as a teleost reference for deeper comparative neuroscience investigations.
a Estimates of snRNA-seq cell-types15 in ST spots by cell2location. Representative tissue hemispheres are ordered counter-clockwise as in Fig. 1 and each spot on the tissue is color-coded by the cell-type with the greatest number of predicted cells within the spot (color-coding scheme is shown in b). Cell-type abundance estimates in a representative tissue hemisphere (S1C2R) are shown with estimated cells as small dots within spots (larger circles). b Cell-type composition of annotated anatomical regions (color represents proportion of cell-types composing anatomical regions). Naming convention for snRNA-seq cell-types is outlined in Johnson et al. 202315. c Anatomical distribution of 5_GABA and 4_GABA cell-types in S1C4R and S1C3R respectively. Spots are colored by the 5_GABA and 4_GABA cell-type with the greatest number of predicted cells within the spot and are otherwise colored gray. Small dots within spots represented predicted 5_GABA and 4_GABA cells.
Conserved telencephalic cell-types in teleosts and mammals
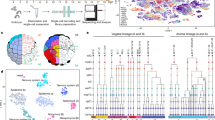
Around 350–450 million years have passed since the last common ancestor of teleost fish and mammals36. Despite a vast array of studies aimed at disentangling the putative mammalian homologues of forebrain regions in fish, obstacles such as the ‘everted’ teleost pallium have made this challenging. To investigate the evolution of cell-types within the telencephalon, we analyzed conserved transcriptional signatures present in comparable cichlid15 and mouse37 forebrain datasets. To conduct these comparisons, previous studies7,8,9 have correlated the expression of common one-to-one marker genes. Recently, a novel integrative approach, SAMap, has been designed for comparisons of cell-types from distantly related species, accounting for protein sequence divergence. We found that SAMap performed better when comparing cell-types from a downsampled dataset to the original underlying dataset37 (SAMap R2 = 0.436; Pearson’s correlation; Fig. 4a) and was therefore primarily used to compare cichlid and mouse telencephalic cell-types (Fig. S9, Supplementary Note 3). Sequence similarity-aware integration of these datasets projected nuclei/cells together in UMAP space (Fig. 4b), despite the vast evolutionary distances between these species. Next, a similarity score was calculated based on the mean k-nearest neighbors between cichlid and mouse cell-types and the score was compared to permutations (Fig. 4c; Supplementary Data 6). Cell-type pairs with similarity scores greater than all permutations (qperm = 0; nperm = 1000) are outlined below, with particular attention paid to pairs where both cell-types were reciprocal top hits.
a A direct comparison of methods for comparing distantly related scRNA-seq datasets suggested SAMap had greater accuracy than correlating shared marker genes (see Methods). b SAMap projects telencephalic cichlid nuclei (n = 33,674)15 and mouse cells (n = 100,531)37 together in UMAP space (cichlid nuclei are ordered to the front). c Similarity scores between cichlid (y-axis) and mouse cell-types (x-axis) with crosses denote reciprocal top hits and dots denoting significance determined by permutation testing (qperm= 0, nperm = 1000). Colored axis labels represent the predicted/probable region of origin of nuclei/cells. AMG=amygdala, CTX=cortex, OLF=olfactory, HIP=hippocampus, PAL=pallidum, STR=striatum, TEL=telencephalon. d Distribution of the percent of conserved markers for significant (qperm=0, dark green) and non-significant (qperm > 0, light green) cell-type pairs. The former had significantly more conserved marker genes than the latter (Welch Two Sample t test; p = 3.79e-49, t = 19.6). e Violin plot of the number of genes driving effects for significant (dark green) versus non-significant (light green) cell-type pairs. Dots indicate the mean value and vertical lines represent the standard error. Possible outliers (<1st or >99th percentile) are not visualized. Cell-type pairs with significant similarities also had significantly more genes driving effects than other cell-type pairs (Welch Two Sample t test; p = 2.13e-30, t = 13.8). f Scaled expression of DEGs driving select cell-type relationships (ngenes = 529). Cell-type pairs with significant similarity are in the same order on the y-axis and genes driving these relationships are in the same order on the x-axis.
We found strong cross-species correspondence of major cell-type classes, with glutamatergic, GABAergic, and non-neuronal populations demonstrating significant transcriptional similarities between cichlid and mouse (Fig. 4c). For example, strong correspondence was observed between oligodendrocyte cell-types (cichlid plp1b + 2.2_Oligo with mouse Plp1 + MFOL1) and oligodendrocyte precursor cell-types (cichlid olig2 + 2.1_OPC with mouse Olig2 + COP1). Cichlid fabp7 + /slc1a3a+ radial glia populations (1.1_RG and 1.2_RG) resembled a mouse astrocyte cell-type (Fabp7 + /Slc1a3 + ACTE1) and 1.1_RG additionally bore similarity to mouse dentate gyrus radial glia (RGDG). In the teleost telencephalon, radial glia line the pallial and subpallial ventricular zones (VZ), which resemble the main neurogenic niches of the adult mammalian brain, the dentate gyrus, and subventricular zone38. In teleosts, radial glia function as stem cells, giving rise to new neurons and glia throughout adulthood38 and are considered astroglial due to their expression of classical astrocyte markers39 despite lacking the stellate morphology characteristic of mammalian astrocytes. Additionally, the teleost pallial VZ contains a population of non-glial proliferative progenitors largely composed of neuroblasts40. Cichlid 9.5_Glut, putatively concentrated along the pallial VZ, expressed markers of neuroblasts (sox4, sox11, mex3a, and zeb2) and bore molecular similarity to Sox4+ granule neuroblasts of the hippocampal dentate gyrus (DGNBL1). The most abundant neuroblast population in the teleost telencephalon forms a rostral migratory stream-like structure in the subpallial VZ, migrating into the OB38 similar to observations in the subventricular zone of rodents41. Indeed, the cichlid cell-type which mapped to the glomerular/mitral layer (gml) of the OB (eomesa + /tbx21 + 15.6_Glut) resembled mouse glutamatergic OB neuroblast populations (Eomes + OBNBL1). Cichlid tp73 + 15.7_Glut and cacna2d2 + 14_Glut, which were similar to several mouse neuroblast populations, additionally resembled dentate gyrus Trp73 + /Cacna2d2+ Cajal-Retzius cells. In the mouse brain, these cells play critical roles in hippocampal and neocortical development and radial glia migration42.
In the mammalian brain, GABAergic neuronal lineages are derived largely from ganglionic eminence progenitor domains (e.g., medial ganglionic eminence, MGE; lateral ganglionic eminence, LGE). Early subpallial territories in the embryonic teleost brain have been proposed as MGE/pallidal-like or LGE/striatal-like based on regionalized expression of neuroregulatory genes42. We observed a reciprocal top hit between cichlid six3a + 4.2_GABA and a mouse LGE-derived striatal MSN population, Six3 + MSN2 (Fig. 4c). In addition, several other cichlid 4_GABA cell-types, which mapped to the Vs and Vd were significantly similar to mouse LGE-derived populations (MSN1-4). Consistent with our results, recent cross-species comparisons between the teleost and mouse forebrain also revealed strong correspondence between mammalian MSNs and subpallial cell-types expected to be located near the Vd in both zebrafish12 and goldfish23. We found that LGE-derived cell-types putatively located in the cichlid and mouse OB mapped to each other with great specificity (etv1 + 5.2_GABA to Etv1 + OBDOP2). Additionally, we observed strong molecular conservation between cichlid GABAergic cell-types and mouse MGE-derived inhibitory interneurons, including sst1.1 + , npy + 15.3_GABA, putatively Vl-derived, with mouse Sst-class interneurons (TEINH21) and between pvalb6 + /nxph1 + 6_GABA with mouse interneurons in the hippocampus and cortex (Pvalb + , Nxph1 + TEINH17-18). A similar finding was reported by ref. 23, which found that goldfish cell-types located in the lateroventral subpallium (referred to by authors as the Vsst) demonstrated strong transcriptional similarity to mouse SST interneurons. Finally, several cichlid cell-types which mapped to the Vv resembled mouse cell-types of the septal nuclei (lhx6a + 15.4_GABA with Lhx6 + TEINH1; zic1 + 15.2_GABA with Zic1 + TEINH2).
Due to the abundance of glutamatergic cell-types and their relative similarity, below we describe statistically significant cell-type relationships across species with less attention to reciprocity. Multiple populations which mapped to the Dm (10_Glut) and Dp (posterior division of D; 11_Glut and 8.7_Glut) bore similarity to a mouse cell-type in cortical entorhinal superficial layers (TEGLU5) as well as to piriform pyramidal cells (TEGLU15-17). Cichlid bhlhe22 + 8_Glut cell-types putatively located in the ventral zone of the lateral subdivision of D (Dl-v), referred to here as Dl-vv (8.5_Glut and 8.9_Glut) were transcriptionally similar to a mouse hippocampal CA3 cell-type (Bhlhe22 + TEGLU23; Fig. 4c). We also observed similarity between several cckb + 8_Glut cell-types which mapped to the Dl-v (8.3_Glut, 8.4_Glut, 8.10_Glut, 8.11_Glut) and mouse cell-types of the subiculum (TEGLU13-14), a mammalian structure in the retrohippocampal formation that mediates hippocampal-cortical communication43. In line with this result, recent studies in zebrafish12 and goldfish23 also reported molecular similarity between the mouse subiculum and cell-types located in the Dl-v. Notably, predicted Dl-g (granular zone of Dl) cichlid rbfox3b+ cell-types, including 8.1_Glut and 12_Glut (Fig. S10), were transcriptionally similar to excitatory cortical projection neurons from cingulate/retrosplenial areas (Rbfox3 + TEGLU6 and TEGLU9). The robustness of these results was supported by analyses of shared marker genes and SAMap driving genes (Fig. 4d–f, Supplementary Note 4, Supplementary Data 7) and by additional comparisons performed using other teleost (goldfish) and mouse telencephalic datasets (Supplementary Notes 5, 6, Figs. S11, 12). Remarkably, the predicted dorsal-to-ventral organization of teleost neuronal populations that were transcriptionally similar to mammalian retrosplenial/cingulate, subiculum, and CA3 neuronal populations reflected the arrangement of these brain regions in mammals44. Taken together, our analyses reveal a suite of glutamatergic neuronal populations in teleosts bearing transcriptional and anatomical similarities to those populating mammalian cortical regions.
Conserved telencephalic cell-types in teleosts and tetrapods
Across vertebrate lineages, the telencephalon demonstrates both commonalities shared between taxa as well as marked, specialized differences and many hypothesized brain region homologies in non-mammalian vertebrates are unclear and actively debated. To further investigate conserved telencephalic populations in vertebrates, we performed cross-species comparisons between cichlid cell-types and cell-types from tetrapod forebrain regions (Fig. 5a–c; Supplementary Data 8), including the axolotl telencephalon9, turtle pallium7, and songbird HVC, RA, and Area X regions8. The HVC and RA are involved in songbird vocal circuits and located in the DVR, a sauropsid pallial structure8. Following the same approach previously described, SAMap was used to integrate cichlid nuclei with datasets from each species separately, revealing several consistent patterns outlined below.
Pairwise transcriptional comparisons of cichlid telencephalic cell-types to axolotl9 (a), turtle7 (b), and songbird8 (c) cell-types using SAMap. Cell-type pairs with higher similarity scores indicate greater transcriptional similarity, crosses denote reciprocal top hits, and dots denote similarity scores greater than all permutations (qperm = 0). Turtle cell-types are colored by their inferred region of origin with the following additional labels: U=unknown, UA=unassigned.
Our comparisons supported correspondence of major non-neuronal cell-types, including cichlid astroglia-like radial glia cell-types (fabp7 + /slc1a2 + 1.1_RGC and 1.2_RGC) to axolotl ependymoglia (Fig. 5a) and songbird astrocytes (Fig. 5c). Cichlid microglia (1.3_MG; csf1r+) and oligodendrocytes (2.2_Oligo; plp1a + ) consistently mapped to their counterparts in all vertebrate comparisons (Fig. 5a–c) and oligodendrocyte precursor cells (2.1_OPC, olig2 + ) showed one-to-one mapping in songbirds. As with the comparison to the mouse telencephalon, sox4 + 9.5_Glut demonstrated significant similarity to neuroblasts in all comparisons. Taken together, these findings point towards strongly conserved molecular signatures present in major non-neuronal populations across vertebrates.
Cichlid GABAergic cell-types demonstrated consistent patterns across comparisons with other vertebrates (Fig. 5a–c; Fig. S13). Cichlid 5_GABA cell-types that mapped to the OB resembled olfactory LGE-derived cell-types (axolotl GABA1 and GABA3; turtle i01) and songbird GABA-1-1, which bore similarity to mammalian non-neocortical LGE-class neurons according to previous analysis8. Cichlid meis2 + , six3a + 4_GABA cell-types similar to mouse striatal MSNs also resembled songbird MSNs from Area X and LGE-derived cell-types in axolotl (GABA11) and turtle (i05). In line with results from our cichlid-mouse analysis, we observed strong transcriptional similarity between vertebrate MGE-derived populations and cichlid pvalb6 + , nxph1 + 6_GABA (turtle i12; songbird GABA-3; axolotl GABA2 and GABA6) and sst1.1 + , npy + 15.3_GABA (axolotl GABA17 non-reciprocally; turtle i10; songbird GABA-2).
Additionally, we found evidence of conserved molecular features in several glutamatergic cell-types (Fig. 5a–c). Similar to our cichlid-mouse comparison, a subset of 8_Glut cell-types predicted to populate the Dl-v (8.5_Glut and 8.9_Glut) consistently grouped with hippocampal-like cell-types in vertebrate datasets, including reciprocal top hits between cichlid 8.5_Glut and turtle e34 from the dorsal medial cortex (DMC) and cichlid 8.9_Glut with axolotl GLUT7. Cichlid Dc (central division of D) cell-type 9.8_Glut was a reciprocal top hit with a turtle population in the posterior dorsal ventricular ridge (pDVR; e25), reported as similar to the mammalian pallial amygdala7. Other cichlid cell-types in the Dc and neighboring Dm-2r (rostral part of subdivision 2 of Dm; 13_Glut) were transcriptionally similar to the pDVR. A cell-type putatively located in the Dp (11.3_Glut) strongly resembled a turtle cell-types in the anterior lateral cortex (aLC; e12), the putative reptilian homologue of the piriform cortex7. Two cichlid cell-types that mapped to the Dl-g had reciprocal top hits with populations in the turtle anterior dorsal cortex (aDC; 8.2_Glut with e16; 12_Glut with e14), and a third cichlid cell-type was also transcriptionally similar to an aDC cell-type (8.8_Glut with e16). One of these cichlid cell-types (12_Glut) was also transcriptionally similar to a cell-type in the turtle anterior dorsal ventricular ridge (aDVR, 12_Glut with e01). Interestingly, several Dl-g cell-types were transcriptionally similar to axolotl cell-types that were previously determined to be transcriptionally similar to cell-types in turtle aDC and aDVR and to mouse cell types in retrosplenial/cingulate cortex and other cortical regions (8.1_Glut with axolotl GLUT2; 8.2_Glut with axolotl GLUT2, GLUT20; 12_Glut with axolotl GLUT22)8,9. These axolotl clusters were present in microdissections of dorsal pallium (GLUT2, GLUT20), medial pallium (GLUT2, GLUT20), and lateral (including ventral) pallium (GLUT2, GLUT20, GLUT22)9.
The robustness of these relationships was evidenced by significant cross-species cell-type pairs exhibiting more shared marker genes (Welch Two Sample t test; axolotl p = 2.17e-10; turtle p = 5.54e-25; songbird p = 1.63e-19; Fig. S14; Supplementary Data 9) and SAMap driving genes (Welch Two Sample; axolotl: p = 1.79e-10, t = 7.08; turtle: p = 1.69e-11, t = 7.27; songbird: p = 5.68e-20, t = 10.49; Fig. S14; Supplementary Data 9) compared to non-significant cell-type pairs (genes that defined the most transcriptionally conserved cell populations across vertebrates are provided in Figs. S15, 16; Supplementary Data 10). Taken together, our analyses reveal strong molecular conservation of forebrain cell-types across vertebrate lineages.
Transcriptional similarities of anatomical regions between teleosts and tetrapods
Our atlas of the cichlid telencephalon provides a powerful tool to explore transcriptional similarities of telencephalic regions across species, amidst competing hypotheses about teleost-to-mammal brain region homologies. Using SAMap, we integrated ST cichlid data with anatomically-annotated scRNA-seq data from turtle pallial microdissections7 and separately with ST data from the mouse brain45. We then used these anatomically-rooted transcriptional data to more directly compare evolutionary relationships of brain regions across vertebrate species. Below we outline significant cross-species relationships between brain regions with particular attention to reciprocal top hits that were additionally supported by cell-type relationships described earlier.
Several cichlid brain regions were transcriptionally similar to turtle pallial brain regions: Dl-vv and the DMC, Dp and the aLC, Dm-2r and the pDVR, and the Dl-g and the aDVR (Fig. 6a; Supplementary Data 11). The relationship between the Dl-vv and the DMC was supported by our cell-type analysis: Dl-vv cell-type 8.5_Glut was a reciprocal top hit with a DMC cell-type (e34), and was additionally transcriptionally similar to several more (Fig. 5b) as well as to mouse hippocampal CA3 cell-type TEGLU23 (Fig. 4c), consistent with ref. 7. Similarly, the reptilian aLC and cichlid Dp have previously been reported as putative homologues of the mammalian olfactory (piriform) cortex7,43 and their homology to each other was further supported by our cell-type comparison (cichlid 11.3_Glut with turtle e12; Fig. 5b). The relationship between Dm and pDVR was supported by cichlid cell-types putatively located in the ventromedial Dm (Dm-2r, Dm-2c (caudal part of subdivision 2 of Dm)) showing significant similarity to turtle pDVR cell-types (13_Glut with e25; Fig. 5b). Notably, both regions have been previously and independently compared to the mammalian pallial amygdala7,46. Interestingly, Dl-g was transcriptionally similar to turtle aDVR but not to turtle aDC. Although several cichlid Dl-g cell-types (8.2_Glut, 8.8_Glut, 12_Glut) were most transcriptionally similar to aDC cell-types (e14 and e16), Dl-g cell-type 12_Glut was also transcriptionally similar to aDVR cell-type e01 (Fig. 5b).
a Transcriptional comparisons of annotated cichlid telencephalic ST anatomical regions to anatomical annotations of turtle pallial scRNA-seq data7 using SAMap. Greater similarity scores indicate greater transcriptional similarity, crosses denote reciprocal top hits, and dots denote significance (qperm = 0). b Transcriptional comparisons of annotated cichlid telencephalic ST anatomical regions to Allen Brain Atlas (ABA) region annotations of mouse ST data45 using SAMap. AMG=Amygdala, HIP=Hippocampal formation, ISO=Isocortex, OLF=Olfactory areas, STR=Striatum, PAL=Pallidum, SEP=Septum and TEL=Telencephalon. c Summed normalized expression of genes driving select significant relationships between cichlid and turtle anatomical regions (turtle aLC and cichlid Dp; turtle DMC and cichlid Dl-vv; turtle pDVR and cichlid Dm-2r; turtle aDVR and cichlid Dl-g). d Summed normalized expression of genes driving select significant relationships between cichlid and mouse anatomical regions (mouse MOB and cichlid OB gc; mouse ACB and cichlid Vd-r; mouse LSX and cichlid Vv; mouse CA3 and cichlid Dl-vv; mouse VIS and cichlid Dl-g).
These results largely aligned with comparisons between cichlid and mouse brain regions. Consistent with previous teleost studies, we observed strong similarities between cichlid and mouse subpallial regions: cichlid OB gc with the main olfactory bulb (MOB), the Vd with the striatum (Vd-r (rostral part of Vd) with nucleus accumbens, ACB), and the Vv with the lateral septal complex (LSX) (Fig. 6b; Supplementary Data 11). Interestingly, cichlid-mouse comparison of pallial regions revealed that the Dl-vv, a densely populated subregion at the ventral-most pole of Dl, bore strong similarity to the CA3 subregion of the hippocampus, consistent with previously proposed relationships between the teleost Dl-v and mammalian hippocampal pallium5,43,46,47. Expression of prox1 in the zebrafish Dl-g has led to a proposed homology between this region and the mammalian hippocampal dentate gyrus18, but this gene was not strongly expressed in cichlid Dl-g or other cell-types. Further, we did not find transcriptional similarity between Dl-g and mammalian dentate gyrus, but instead found that Dl-g was most transcriptionally similar to the mammalian visual cortex (VIS, Fig. 6b), complementing our earlier reported transcriptional similarities between Dl-g cell-types (8.1_Glut and 12_Glut) and were significantly similar to mouse neocortical cell-types (TEGLU6, TEGLU9; Fig. 4b). Additionally, the cichlid Dp was most transcriptionally similar to the mouse piriform cortex (PIR), though it did not meet our significance threshold. This similarity agrees with the above comparisons to turtle brain regions and with the partial eversion model of the teleost telencephalon2. These relationships were reinforced by additional analyses of shared marker genes and SAMap driving genes (Fig. 6c, d, Fig. S17, Supplementary Notes 7, 8, Supplementary Data 12).
Anatomical subregions in vertebrates exhibiting transcriptional similarity to the mammalian cortical regions
The teleost brain lacks a layered cortex and telencephalic populations that resemble those present in this elaborated mammalian structure remain a matter of debate. A popular theory casts doubt on the existence of a cortex-like homologue in fish5, while others propose the Dc2,31 or the Dl-d(dorsal subdivision of D)/g region as putative phenotypic homologues of the dorsal pallium, which gives rise to the neocortex and mesocortex in mammals2,3,48,49. Similarly, the homologies of specific reptilian brain regions to mammalian cortical regions are the subjects of ongoing debate7. We find transcriptional similarity between predicted Dl-g cell-types to mouse neocortical populations (Fig. 4c) and cell-types from the turtle aDC and aDVR (Fig. 5b). Previous analyses found that the turtle aDC and aDVR possessed cell-types with significant correlations to the mammalian neocortex using all genes, but only cell-types in the aDC had significant correlations when the analysis was restricted to transcription factors7. To gain further insight into the transcriptional relationships between these structures, we used SAMap to compare turtle7 and mouse37 scRNA-seq datasets.
This analysis revealed significant and reciprocal relationships between both an aDC cell-type (e07) and an aDVR cell-type (e01) to mouse neocortical cell populations (TEGLU4 and TEGLU9 respectively) (Fig. 7a; Fig. S18). While significant and reciprocal, these turtle cell-types had similarities to other mammalian cell-types that were not neocortical. The same is true of other significant, but non-reciprocal similarities were observed aDC and aDVR cell types and other mouse cortical and non-cortical populations (Fig. 7a; Fig. S18), consistent with previous reports that turtle aDC and aDVR contain cell-types that are transcriptionally similar to mammalian neocortex7. Interestingly, this previous work also found that the relationship between aDVR and neocortex, but not between aDC and neocortex, was no longer present when only transcription factors were analyzed, raising the possibility that some transcriptional similarities may be driven by specific gene classes (e.g., transcription factors). To investigate this, we tested whether the composition of different gene classes (using curated lists7,15 of transcription factors, neuromodulatory ligands, neuromodulatory receptors, or other; see Methods; Supplementary Data 13) among SAMap driving genes differed across the different transcriptional relationships we identified among cichlid, turtle, and mouse brain regions. This analysis revealed that the composition of gene classes among SAMap driving genes did not differ across cell-type/brain region similarities for turtle-mouse comparisons, cichlid-mouse comparisons, or cichlid-turtle comparisons (Fig. 7b–d, Supplementary Data 13, Supplementary Note 9). Taken together, our results are largely consistent with previous work, but support the idea that cross-species cell-type and brain region transcriptional similarities are driven by diverse functional categories of genes.
a Transcriptional comparison of cell-types in the turtle and mouse forebrain using SAMap. Results for cell-types from the turtle aDC and the aDVR7 to cortical mouse cell-types37 are shown (full results can be found in Fig. S18). b–d Composition of gene categories driving significant cichlid-mouse, cichlid-turtle, and turtle-mouse cell-type pairs (transcription factors, neuromodulatory ligands and receptors). No significant differences in composition were found. e Simplified schematic demonstrating the arrangement of pallial zones, supported by our data and results, in representative coronal sections of the adult mouse (modified from ref. 79) and cichlid forebrain resulting from the developmental processes of neural tube evagination and eversion, respectively. Selected cichlid and mouse37 cell-type pairs shown on the right demonstrate significant transcriptional similarity and support a model of partial eversion2 of the teleost pallium. Colors represent the dorsal, medial, lateral, and ventral pallium divisions (separated by black lines). Teal=dorsal pallium (DP), red=ventral pallium (VP), navy blue=medial pallium (MP), orange=lateral pallium (LP) and gray=subpallium. Gray lines represent subregions of the teleost telencephalon.
Based on these results, together with our earlier findings and previous literature, we propose the following brain regions contain molecularly conserved cell-types in cichlids, turtles, and mice: Dl-g, aDC, and mesocortex (retrosplenial/cingulate cortex, subiculum)49; Dl-vv, DMC, and hippocampus (CA3); Dm-2r, pDVR, pallial amygdala; Dp, aLC, and piriform cortex. These findings support the arrangement of putative pallial divisions outlined in the partial eversion model, which proposes the Dl-v as medial pallial, the ventromedial Dm as ventral pallial, the Dp as lateral pallial, and the dorsal Dl as dorsal pallial2 (Fig. 7e).
Discussion
Here we create a spatially-resolved molecular atlas of the Mchenga conophoros telencephalon using complementary techniques, snRNA-seq and ST, to investigate cell-types sequenced at single cell resolution within tissue architecture. We find remarkable correspondence between transcriptional profiling and neuroanatomy, whereby both ST clusters and snRNA-seq cell-types align strongly with well-described neuroanatomical subregions of the fish telencephalon, consistent with previous observations in the mouse brain33,45. Using a comparative approach that considers protein sequence similarity between species, we identify a suite of non-neuronal and neuronal populations that show conserved patterns of gene expression across all major vertebrate lineages. By comparing both cell-types and regions across species, we find support for widely-accepted as well as controversial evolutionary relationships between specific teleost and mammalian brain regions, including a putative teleost homolog for mammalian mesocortex.
Our results are consistent with previous work in goldfish23 and zebrafish12. Although differences in dissections, clustering, and cell-type/region annotations between studies can introduce noise to cross-species mapping, our comparative analyses reveal strong correspondence of spatially-resolved cell-type atlases between cichlids and goldfish, including strong transcriptional conservation of GABAergic cell types mapping to similar subpallial domains in both species, aligning with a previous study of the zebrafish telencephalon12. Similarly, despite differences in anatomical annotations of the cichlid and goldfish pallium, a suite of glutamatergic cell-types also demonstrate strong transcriptional conservation. In cichlid, goldfish, and zebrafish studies, similarities were found between cell-types in the Dl-v to mammalian populations in the retrohippocampal formation, including the subiculum, a relationship we discuss more below. Future studies across the diversity of teleost lineages, incorporating ST, are needed to trace the evolution of cell-types and brain regions in this diverse group.
We find support for homologies between specific cichlid GABAergic populations and mammalian MGE-derived Sst+ and Pvalb+ interneuron classes and LGE-derived populations, including OB cell-types and striatal MSNs. Cichlid MSN-like cell-types, like transcriptionally similar goldfish MSN-like cell-types23, were transcriptionally similar to mouse MSN cell types and anatomically mapped to the Vd, a region which receives substantial dopaminergic input and is considered homologous to the mammalian nucleus accumbens of the striatum1,29. Additionally, these cell-types were transcriptionally similar to goldfish GABAergic MSN-like cell-types which mapped to the Vd, further strengthening the comparison between this region in teleosts to the mammalian striatum. Our results also support a previously proposed homology between the cholinergic Vv and the tetrapod septum50. Various studies have shown that both the striatum and septum, and their putative homologues in fish, play important roles in mediating evolutionarily-relevant social behaviors50,51,52,53. Our results strongly suggest that core cell-types in these subpallial regions are conserved across vertebrates, perhaps due to essential behavioral functions.
Our results also support the partial eversion model for teleost pallial organization2. The pallium has presented perhaps the most challenging puzzle in vertebrate brain evolution due to its markedly unique adaptations across vertebrate clades. The teleost telencephalon exhibits an everted morphology, but the extent of eversion is unclear. Some models suggest a complete eversion of all pallial zones, while others propose only a partial eversion2,3,4,5,6. Consequently, these models differ in the teleostean equivalents of mammalian dorsal, lateral, and ventral pallium divisions, but agree that the medial (hippocampal) pallium is displaced to a dorsolateral location in fish43. Disagreement between eversion models is rooted partly in the caudolateral position of the Dp, which is the main pallial recipient of secondary olfactory input in the fish telencephalon43 and is thus the putative homologue of the mammalian lateral (olfactory) pallium. Indeed, studies in zebrafish reveal functional roles for the Dp in odor-evoked activity similar to those performed of the mammalian piriform cortex54,55. The altered position of the Dp relative to the Dl-v is accounted for in the partial eversion model2. In support of this model, we reveal transcriptional similarity between the cichlid Dp and its associated cell-types and the mammalian piriform cortex populations. Furthermore, these cichlid populations also resemble the turtle aLC and its corresponding cell-types, considered the reptilian homologue of the piriform cortex7. These data are compelling evidence for molecular conservation of pallial olfactory populations across vertebrates.
Support for the partial eversion model also arose from our analysis of Dm. In mammals, the ventral and dorsal pallial divisions give rise to the pallial amygdala and neocortex, respectively, both of which are highly complex structures whose homologues in other vertebrates have been subjects of intense debate. For example, the sauropsid dorsal venrticular ridge (DVR) has been proposed as a possible homologue of mammalian isocortex, but is a ventral pallial structure and therefore has been increasingly viewed as pallial amygdalar56,57. Similarly, the teleost Dm has been compared to both the dorsal and ventral pallial divisions of the tetrapod brain2,58. The latter stems in part from a demonstrated role of the Dm in emotional learning and behavior58 and critically, these experiments largely targeted the ventromedial portion of Dm positioned along the midline59. Furthermore, connections of the ventromedial Dm are comparable to those of the pallial amygdala, whereas the dorsal Dm receives ascending sensory input from the preglomerular complex resembling mammalian thalamic-neocortical pathways59. The aforementioned partial-eversion model accounts for these observations, proposing the ventromedial Dm as ventral pallial and the remaining Dm as dorsal pallial. Consistent with this model, we find both cell-type and region transcriptional similarity between ventromedial Dm (Dm-2r) and turtle pDVR, which previous work found to be transcriptionally similar to the pallial amygdala7.
The hippocampus, functions in memory, spatial navigation and learning60 and is generally considered to be highly conserved across vertebrates, although specific subregion homologies are debated61. Behavioral studies in goldfish have supported the ventral subdivision of the dorsolateral pallium (Dl-v) as a possible hippocampal homolog, demonstrating that it is necessary for similar learning, memory, and behavioral functions as the mammalian hippocampus47,62,63. Our results support this homology, as the ventral-most Dl-v region and its corresponding cell-types exhibited striking transcriptional similarities to cell-types in mammalian hippocampal CA3 region, and we additionally observed transcriptional similarities between this region and both hippocampal CA3 and the dentate gyrus, both of which have been previously been implicated in social and spatial information processing64,65.
Our results additionally support teleost homologs of mammalian mesocortical neuronal populations. In the mammalian brain, the retrosplenial cortex is positioned between the 6-layer neocortex and the hippocampal formation and forms reciprocal connections with the subiculum, the hippocampus, and cortical regions66,67,68, including the visual cortex69. Due to its unique anatomical position, the subiculum and retrosplenial cortex are often classified as mesocortex49, a transitional cortex that develops from the embryonic dorsomedial pallium70. We find strong transcriptional similarities between cichlid Dl-g/Dl-v cell-types and mammalian retrosplenial/subiculuar cell-types, and these relationships were further reinforced by comparisons to a second mouse brain scRNA-seq dataset71. Strikingly, we find that the anatomically sequential (dorsal-to-ventral) organization of populations transcriptionally similar to mouse retrosplenial cortical, subicular, and hippocampal populations reflects the arrangement of these regions in the mouse brain. Taken together, these results further support the partial eversion model, and support the idea that specific neuronal populations in teleost Dl-g/Dl-v and mammalian mesocortex are homologous.
The relationships between cichlid Dl and mammalian retrosplenial/subiculuar/hippocampal regions are particularly interesting given our recent work supporting a central role for Dl in cichlid bower-building behavior, a courtship behavior involving the spatial manipulation of sand15. Two cell-types spatially mapping to Dl-g and Dl-v (8.1_Glut and 8.4_Glut, respectively) exhibited building-associated changes in their relative proportions. Behavior-associated signatures in a radial glial subpopulation bordering Dl, together with cell-cell communication analyses, supported a circuit model in which multiple neuronal populations and glia together coordinate the cellular reorganization within Dl during building. Here we find transcriptional similarities between 8.1 Glut and a mouse retrosplenial/cingulate layer 2-derived cell population (TEGLU6), and between 8.4_Glut and a mouse subiculum-derived cell population (TEGLU13). Previous work demonstrates a functional role for the retrosplenial cortex and subiculum in mammalian spatial/sociospatial learning and memory72,73. In light of these observations and our results, it is intriguing to speculate that bower -building is regulated by conserved mesocortical-like cell populations and circuits that encode representations of the social and spatial environment.
The evolutionary origins of the mammalian cortex have been intensely debated. One center of attention has been the evolutionary relationships between the aDC and DVR in reptiles and the mammalian cortex7. Interest in the DVR as a possible cortical homolog grew through repeated observations that it shared similar molecular, connective, and functional properties with the mammalian cortex56,74. Importantly, however, the mammalian cortex develops from the dorsal pallium, whereas the DVR develops from a distinct ventral pallial zone that exhibits divergent neurodevelopmental transcriptomic trajectories10,75,76. In contrast, the reptilian aDC arises from the dorsal pallium, and also bears striking molecular, connective, and functional similarities with the mammalian cortex7. For these reasons, the aDC is increasingly considered to share evolutionary origins with the mammalian cortex57. Interestingly, our analyses echoed the cortical signatures of both regions. Although our cross-species cell-type and region mapping usually converged, one exception was that Dl-g cell-types were more transcriptionally similar to aDC (turtle) and retrosplenial (mouse) cell-types, whereas the Dl-g region was more transcriptionally similar to aDVR (turtle) and visual cortex (mouse). Factors that may cause divergence between cell-type and region mapping include the evolution of distinct cell-types within a brain region in one or both lineages, variation in how brain regions and subregions are defined in relation to lineage-specific patterns of cellular organization, and technical differences across studies (e.g., sequencing methodology, region annotation, etc.). In addition to cell-type mapping, the immediate proximity between Dl-g and other hippocampal-like regions and cell-types mirrors the immediate proximity between aDC and other hippocampal-like regions and cell-types in reptiles, and furthermore is consistent with the partial eversion model. Taken together, these results support cichlid Dl-g as dorsal pallial, although striking transcriptional similarities to the reptilian ventral pallial aDVR are apparent, consistent with previous work7,8,10.
In summary, our work demonstrates deep cell-type and region homologies in the vertebrate forebrain and addresses several knowledge gaps surrounding vertebrate brain evolution. Perhaps most notably, we find support for a partially everted telencephalon in teleosts compared to mammals, a pallial amygdala-like ventral Dm subdivision, and a dorsal pallial Dl-g region containing mesocortical-like neuronal populations similar to those populating the reptilian aDC and the mammalian subiculum and retrosplenial/cingulate cortex. Future comparative studies will deepen our understanding of cell-type and brain region evolution among teleosts and across vertebrate lineages.
Methods
Subjects
Male Mchenga conophoros cichlids used in this study were fertilized and raised into adulthood (>180 days) in the Georgia Institute of Technology (Atlanta, GA) Engineered Biosystems Building cichlid aquaculture facilities. All procedures were approved by the Institute Animal Care and Use Committee at the Georgia Institute of Technology (IACUC protocol number A100029). We have complied with all relevant ethical regulations for animal use. All animals were collected as fry from mouthbrooding females approximately 14 days post-fertilization and raised on a ZebTec Active Blue Stand Alone system until approximately 60 days post-fertilization, at which point animals were transferred to 190-L (92 cm long × 46 cm wide × 42 cm tall) glass aquaria ‘home tanks’ maintained on a central recirculating system. Environmental conditions of aquaria were as follows: subjects were housed in pH = 8.2, 26.7 °C water in social communities (20–30 mixed-sex individuals) and maintained on a 12-h:12-h light:dark cycle (full lights on between 8 am and 6 pm Eastern Standard Time (EST)) and dim lights for 60 min periods between light-dark transition (7 am–8 am and 6 pm–7 pm EST). Subjects were fed twice daily between 8–9 am and 2–3 pm (Spirulina Flake; Pentair Aquatic Ecosystems, Apopka, FL, U.S.A.). Reproductive adult subject males were introduced from home tanks to experimental tanks as described in ref. 15 which contained sand and four reproductive adult size-matched stimulus females of the same species.
Tissue processing
Adult subject males (n = 2) were collected from experimental tanks between 11 am and 2 pm EST (3–5 h after full lights-on) to control for potential effects of food intake and circadian timing on brain gene expression. Subjects were rapidly anesthetized with tricaine immediately following collection, measured for body mass (BM) and standard length (SL), and decapitated for brain extraction. Telencephala were dissected under a dissection microscope (Zeiss Stemi DV4 Stereo Microscope 8x - 32x, 000000-1018-455), in Hibernate AB Complete nutrient medium (HAB; with 2% B27 and 0.5 mM Glutamax; BrainBits) containing 0.2 U/μl RNase Inhibitor (Sigma) to prevent RNA degradation. Immediately following dissection, telencephala were embedded in disposable base cryomolds (7 mm × 7 mm × 5 mm; Simport Scientific) containing chilled Optimal Cutting Temperature (OCT) compound (TissueTek Sakura) and flash-frozen on dry ice. Testes were then surgically extracted and weighed to calculate gonadosomatic index (GSI=gonad mass/BM*100) for each subject (subject information available in Supplementary Data 1). Tissue blocks were stored in 5 ml CryoELITE tissue vials (Wheaton) at −80 °C until further processing.
Telencephala were cryo-sectioned coronally at 10 μm thickness using a Cryostar NX70 cryostat at −20 °C. Four tissue sections per subject were mounted onto 6.5 mm2 capture areas on pre-cooled Visium Spatial Gene Expression slides (10x Genomics, 200233) and slides were stored at −80 °C for further processing. RNA quality of the tissue sections (RIN > 7) was confirmed using the Agilent RNA 6000 Nano Kit on the Bioanalyzer 2100 system (Agilent). Visium spatial gene expression slides were processed according to manufacturer instructions (10x Genomics; Methanol Fixation, H&E Staining, and Imaging – Visium Spatial Protocol CG000160). Slides were warmed to 37 °C for 1 min and fixed in methanol at −20 °C for 30 min, followed by isopropanol incubation for 1 min at room temperature. Tissue sections were then stained for hematoxylin and eosin (H&E). Brightfield images of H&E-stained slides were taken at 1.33 mm/pixel resolution using a Zeiss AxioObserver Z1 Fluorescent Microscope (Zeiss) with a 5X objective and stitched using Zen 2 software (blue edition, Zeiss) prior to library construction.
Visium spatial gene expression library generation
Dual-index Illumina paired-end spatial gene expression libraries were prepared according to manufacturer instructions (10x Genomics; Visium Spatial Gene Expression Reagent Kits User Guide CG000239). Fixed and stained tissue sections were enzymatically permeabilized for 18 min. Optimal permeabilization time was determined to be 18 min based on initial tissue optimization trials (10x Genomics; Visium Spatial Tissue Optimization Reagent Kits User Guide CG000238). Poly-adenylated mRNA released from cells was captured by primers on the underlying spatially-barcoded 55 mm-diameter gene expression spots. Primers include a 10x spatial barcode, unique molecular identifier (UMI) and a partial read 1 sequencing primer (Illumina TruSeq Read 1). Incubation with reverse transcription (RT) reagents produced spatially barcoded, full-length cDNA. Barcoded cDNA was denatured, transferred into tubes, and amplified via PCR. Amplified cDNA was size-selected with SPRIselect. During final library construction, P5 and P7 paired-end construct sequences, i5 and i7 sample indices, and a read 2 primer sequence (Illumina TruSeq Read 2) were added. Quality was assessed using high sensitivity DNA analysis on the Bioanalyzer 2100 system (Agilent). Libraries were pooled together and sequenced on the NovaSeq 6000 (Illumina) platform using the 150-cycle SP Reagent Kit (800 M reads; sample information available in Supplementary Data 1). The raw data was generated by ref. 15 and are publicly available in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) under accession code GSE217615.
Statistics and reproducibility
Spatial transcriptomics data pre-processing and quality control
FASTQ files were processed with 10x Genomics Space Ranger 1.3.1. Reads were aligned to the Maylandia zebra Lake Malawi cichlid genome assembly16 using a splice-aware alignment algorithm (STAR) within Space Ranger and gene annotations were obtained from the same assembly (NCBI RefSeq assembly accession: GCF_000238955.4, M_zebra_UMD2a). H&E images were oriented based on fiducial markers and the appropriate slide layout files were chosen. Space Ranger aligned barcoded spot patterns to the input slide image and distinguished tissue from background on the slide. Following these steps, Space Ranger generated filtered feature-barcode matrices (one per slide) containing expression data for a total of 32,471 features (corresponding to annotated genes) and a total of 6755 barcodes (corresponding to spots). In R the “Seurat” package was used to remove spots with less than one UMI and spots containing small pieces of stray tissue (48 spots removed).
Selection of tissue sections for downstream analysis
This analysis focuses on seven tissue sections from two subject males, S1 (n = 3 sections) and S2 (n = 4 sections). This decision was made based on (1) the high quality of the tissue sections, and (2) the observation that this combination of S1 and S2 sections yielded a comprehensive representation of telencephalic subregions along the rostral-caudal axis. The order of the tissue hemispheres along the rostral-caudal axis was determined through visual inspection of H&E-stained tissue images.
Clustering and selection of parameters
To prevent over-tuning of clustering parameters, a systemic method was used to determine optimal clustering parameters. ChooseR is a tool compatible with Seurat that evaluates clustering quality based on robustness metrics of bootstraps of the data. The code was adapted to evaluate more clustering parameters and to follow a slightly different workflow. The additional parameters that were evaluated were min.dist and n.neighbors in the RunUMAP function from Seurat. To ensure that bootstraps were entirely independent, the find_clusters function was modified to include the SCTransform, RunPCA, and RunUMAP functions from Seurat. Without these functions, there would be information leakage in the form of the variable genes and reduced dimensional space. In SCTransform, tissue sections were regressed out using var.to.regress and the resulting “SCT” assay was set as the active assay. 50 dimensions were used in RunPCA and RunUMAP. Additionally, in RunUMAP spread was set to 1, n.epochs was set to 1000 and metric was set to “euclidean”. In FindNeighbors, reduction was set to “UMAP” and the first two dimensions were used, k.param was set to the same value as n.neighbors, n.trees was set to 500, and prune.SNN was set to 0. In FindClusters, algorithm was set to 2.
ChooseR was used to evaluate the clustering of combinations of min.dist and n.neighbors in RunUMAP and resolution in FindClusters. The set of values tested were as follows: min.dist of 0.1, 0.2, 0.3, 0.4, and 0.5; n.neighbors of 10, 20, 30, 40, and 50; and resolution of 0.2, 0.4, 0.6, 0.8, 1.0, and 1.2. The default value of 80% subsampling of the data was used as well as the default of 100 bootstraps (per combination of parameters). ChooseR defines the near-optimal clustering parameters as the one yielding the highest number of clusters whose median silhouette score is greater than the highest lower bound (95% confidence interval). For our ST data, this methodology identified the near-optimal clustering parameters to be min.dist=0.1, n.neighbors=30, and Resolution=1.2, which we used to cluster spots.
Human Ortholog identification
Teleosts including M.zebra have undergone a genome duplication since their divergence with mammals (last common ancestor est. 360–450 MYA36), thus identification of human orthologs is non-trivial and multiple methods were used to ensure accuracy. Human orthologs to cichlid genes were retrieved from ENSEMBL and ref. 77, which uses a protein-based approach. To perform interspecies correlation analysis, one-to-one orthologs are required. For all orthologs, cichlid genes were kept only where the human ortholog was contained in one of the following: NCBI gene name, NCBI gene symbol, ENSEMBL gene symbol, ref. 77, and human ortholog from ENSEMBL. Due to the genome duplication, there are numerous many-to-one orthologs. In these cases, the cichlid gene with the greatest consistency among the aforementioned sources was kept. In cases of ties, the cichlid gene that contained the greatest number of UMIs in the spatial transcriptomics and single nuclei gene expression matrices was kept. All other cichlid genes were removed. This resulted in 13,237 cichlid genes for comparative analysis of shared marker genes. For interspecies integration analysis, a different method, BLAST, was used for ortholog identification (see ‘Interspecies integration of single cell/nucleus RNA-seq data’).
Computation of cell-type abundance estimation in spatial transcriptomics spots
For cell-type deconvolution of spots, cell2location33 was used as it was among the top performing tools for this task reported by a recent benchmarking paper34. Cell2location is a Bayesian model that estimates the absolute abundance of cell-types at each spot. First, the regression model for the single cell data was initialized with default settings, using batch as the batch_key, and the model was trained using a maximum of 250 epochs. Next, the regression model for the spatial transcriptomics data was initialized with the single cell reference signatures, default settings, and hyperparameters selected based on cell2location’s recommendations. We did not observe strong within-batch variation in total RNA count, therefore we set detection_alpha=200. By manual visual inspection of a subset of spots, we estimated the approximate mean number of cells per spot to be 8 and therefore set N_cells_per_location=8. The model was then trained using a maximum of 30,000 epochs. Finally, the estimated mean abundances of cell-types were rounded and spots with no estimated cells were assigned one cell to the cell-type with greatest abundance.
Manual cell abundance estimation in spatial transcriptomics spots
To evaluate the accuracy of the estimates of cell abundance in spatial spots by cell2location, we compared predicted cell numbers to our manual counts for a subset of spots in a single tissue hemisphere (S1C2R). Spots at the peripheral edges of the tissue contained a high density of cell bodies that could not be reliably distinguished and these spots were therefore excluded from the comparison. The Pearson’s and Spearman’s correlation coefficient was found between manual and computational estimates of cell abundance (Fig. S8).
scRNA-seq dataset retrieval
Mouse (est. divergence time from humans ~112 ± 3.5 Myr78) gene expression data was retrieved from refs. 37,71. Gene expression data for other vertebrate taxa was retrieved from ref. 9 (axolotl, est. divergence time from humans ~360 ± 14.7 Myr78), ref. 7 (turtle, est. divergence time from humans ~222 ± 52.5 Myr78) and ref. 8 (songbirds, est. divergence time from humans ~222 ± 52.5 Myr78). Next, the datasets were filtered to include only cells from the telencephalon. Data from ref. 37 was filtered to include only cells from cell-types annotated as belonging to the telencephalon and data from ref. 71 was only retrieved for the following brain regions: Frontal Cortex, Globus Pallidus, Hippocampus, Posterior Cortex, and Striatum. No filtering was required for the axolotl and turtle datasets, as the cells came from the telencephalon. All cells from the HVC and RA of songbirds were kept, but only MSNs from Area X were retained due to non-descriptive annotations of the other cells. Next, cells annotated by ref. 37 as low-quality were removed. This information was not readily available for ref. 71, thus the dataset was subsetted to include only cells with greater than 500 UMIs, 500 genes, less than 5000 UMIs, and less than 2500 genes. The other vertebrate datasets retrieved were already filtered, so no further filtering was performed. Finally, due to the large number of cells from ref. 71, cells were randomly and evenly removed from the largest clusters until 100,000 cells remained. All datasets were normalized using SCTransform using default options.
Evaluation of methods for comparative cell-type analysis
The accuracy of two methods for comparison of cell-types across species were evaluated: correlation of common marker genes and SAMap (see Methods below). To determine their accuracy, they were tested by comparing cell-types whose relationships are already known. Thus, a scRNA-seq dataset from mouse telencephalon37 was downsampled and compared to itself. Therein, the cell-types in the downsampled dataset are known to be the same as the original. To downsample the dataset, 10% of UMI’s were removed from each cell and three cell-types were randomly chosen to be removed so the comparison was not one-to-one. Then the downsampled dataset and the original dataset were compared using the two methods. The resulting matrices of correlations/scores between cell-types in the downsampled and original dataset were compared to the identity matrix (i.e., same cell-types were set to 1 and others were set to 0). The coefficient of determination (squared Pearson’s correlation) was found between the matrices resulting from the two methods and the identity matrix.
Interspecies correlation of single cell/nucleus RNA-seq data
To compare sc/snRNA-seq from the cichlid and mouse telencephalon, a procedure similar to refs. 7,8,9 was implemented as it is well suited for comparisons of distantly related species. Briefly, for each cell-type or brain region a Spearman correlation and corresponding p value were calculated based on the average normalized expression of DEGs present in both species. For robustness, correlations were calculated using multiple mouse datasets37,71. Interspecies correlations using data from ref. 37 were performed on the “ClusterName” metadata and correlations using data from ref. 71 on the “subcluster” metadata.
DEGs (adjusted p < 0.05, log2FC > 0.25, and min.pct > 0.1) were found in the cichlid and mouse datasets using the FindAllMarkers functions with only.pos = T and otherwise default parameters. Then, the common set of genes that were DEGs in both species was found (see :Human Ortholog identification), and the average expression of these genes was calculated across the whole dataset using the AverageExpression function in Seurat. Similar to ref. 8, these average gene expression values were normalized by log(x + 1) + 0.1 and were divided by the mean for each gene across the dataset. The normalized average gene expression matrices for the cichlid and mouse datasets were then correlated using a Spearman correlation. Significance of these correlations was determined using two criteria: (1) Bonferroni adjusted p-value of Spearman correlation test <0.05 and (2) Bonferonni adjusted p value of permutation test <0.05. The former was calculated using cor.test in R with method = “spearman” and alternative = “greater”. Similar to ref. 7, the latter was found by shuffling the normalized average gene expression values across cell-types in the cichlid gene by cluster matrix, then re-calculating the Spearman correlations with the mouse gene by cluster matrix. 1000 permutations were performed and the p value was calculated by finding the proportion of correlations from the permutations greater than the real, non-permuted correlations.
Interspecies integration of single cell/nucleus RNA-seq data
Sc/snRNA-seq data from different vertebrate taxa were integrated to enable comparative analysis of transcriptional similarities of cell-types. To achieve this we used SAMap13, a python package, as it was designed for comparative analyses of distantly related species. SAMap considers sequence similarity of genes between species in its projection of the datasets in joint lower-dimensional spaces. This method allows genes with greater sequence similarity across species to weigh more heavily in the integration, which is advantageous when comparing species with large evolutionary distances like cichlids and mice. First, to determine sequence similarity, the proteomes of the vertebrate taxa were downloaded from NCBI using the version of the assemblies used to create the sc/snRNA-seq datasets: Maylandia zebra (GCF_000238955.4), Mus musculus (GCF_000001635.27), Chrysemys picta bellii (GCF_000241765.3) and Taeniopygia guttata (GCF_003957565.1). For the axolotl (Ambystoma mexicanum) dataset, the assembly was retrieved from v6.0 release of the genome not located on NCBI (AmexT_v47). Next, blastp was executed to identify reciprocal blast hits between vertebrate taxa and cichlids using SAMap’s map_genes.sh script. Then, the raw gene by counts matrix of the sc/snRNA datasets from their Seurat objects were saved as an h5ad file and processed using the SAMAP function with arguments specifying the gene symbols of each protein and the directory containing the blastp results. Finally, the run function was executed using default parameters.
The knn-graph produced by SAMap was then used to create a similarity score between cell-types in cichlids and other vertebrates. The similarity score was defined as the mean number of k-nearest neighbors between cells from other vertebrates to cichlid nuclei. To determine significance, a permutation test was performed in which the cell-type labels of cells/nuclei for both datasets were shuffled and similarity scores were calculated. This was repeated 1000 times and cross-species cell-type pairs with similarity scores greater than all permutations of each cichlid cell-type were considered significant.
Identification of genes driving cell-type relationships discovered using SAMap
To find the genes driving cross-species cell-type relationships, the function GenePairFinder from SAMap was used with default parameters. For every pairwise combination of cell-types, the find_genes function from SAMap was used to find the genes driving the relationship, with n_genes set to the total number of genes in the datasets. The default parameter returns the top 1000 genes driving the relationship, but we allowed the function to return all the genes it found to drive the relationship. These genes are defined by SAMap as genes that are differentially expressed in each cell-type and contribute positively to the cross-species correlation of the cell-types.
Identification of genes upregulated in conserved cell-types across vertebrates
For strongly conserved cell-types, marker genes (significantly differentially expressed genes with adjusted p < 0.05, log2 fold change >0 and expressed in a minimum of 10% of cells/nuclei) with the same human ortholog in all datasets were found. Strongly conserved cell-types included: OLIG (mouse OEC; bird Oligo; turtle tsOlig; axolotl OLIG15; cichlid 2.2_Oligo), OB (mouse OBDOP2; bird GABA-1-1; turtle i01; axolotl GABA3; cichlid 5.2_GABA), MGE1 (mouse TEINH17; bird GABA-3; turtle i07; axolotl GABA2; cichlid 6_GABA), MGE2 (mouse TEINH21; bird GABA-2; turtle i08; axolotl GABA17; cichlid 15.3_GABA), MSN (mouse MSN1; bird MSN3; turtle i05; axolotl GABA11; cichlid 4.1_GABA), DGNBL (mouse DGNBL1; bird Pre-2; turtle tsNPCs; axolotl NB1; cichlid 9.5_Glut) and CA3 (mouse TEGLU23; bird HVC_Glut-3; turtle e34; axolotl GLUT7; cichlid 8.9_Glut). Additionally, human orthologs in conserved cell-types that were upregulated (positive log2FC value), but not meeting the other criteria of marker genes were found. When multiple genes in a species had the same human ortholog, the gene with the greatest log2FC was selected.
Interspecies comparison of anatomical regions
To gain insight into the similarity of anatomical regions across vertebrates, we compared scRNA-seq data from turtles and spatial transcriptomics data from mice to cichlid spatial transcriptomics data separately. Both analyses were performed in the same manner described in ‘Interspecies integration of single cell/nucleus RNA-seq data’. For the comparison to the turtle dataset, the detailed anatomical labels were used when available and the broad anatomical labels were used in all other cases. The mouse spatial transcriptomics dataset was retrieved from ref. 45 and the dataset was subset to include only cells from the cerebrum (Allen Brain Atlas abbreviation ‘CH’), the ventricular systems (Allen Brain Atlas abbreviation ‘VS’), and fiber tracts (Allen Brain Atlas abbreviation ‘fiber tracts’). Then, the dataset was normalized using SCTransform, and the annotations from ABA were used (ABA_acronym column in the metadata). Since these annotations were extremely detailed, the parent annotation was used in some cases. Moreover, spots annotated as cortex (“CTX”) with no more detail were assigned to “U_CTX” to represent undefined cortical regions.
Composition of genes driving effects and conserved marker genes by transcription factors, neuromodulatory ligands and receptors
The composition of genes driving relationships between cell-type pairs was investigated. The number of genes driving SAMap effects and the number of shared marker genes belonging to the following gene categories were found: transcription factors, neuromodulatory ligands, receptors, or “other”. Transcription factors were obtained from ref. 7 and neuromodulatory ligands and receptors were obtained from ref. 15. The aforementioned lists are of human genes, thus for gene pairs identified by SAMap from non-human species, the human ortholog of the mouse gene was used when possible (i.e., in cichlid-mouse and turtle-mouse comparisons) and otherwise the human ortholog of cichlid genes was used (i.e., cichlid-turtle comparison). The number of genes from each category were found and compared for across cell-type hits. To determine significance, a χ2 test was performed on the mean number of transcription factors, neuromodulatory ligands, and receptors.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data needed to evaluate the conclusions in this paper are present in the paper and/or the Supplementary Materials. The spatial transcriptomics data were generated by Johnson et al. 202315 and are publicly available in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) under accession code GSE217615. Source data for all graphs presented in the main figures can be found in Supplementary Data 14.
Code availability
Custom scripts for data analyses can be found at GitHub (https://github.com/ggruenhagen3/cichlid_st) and Zenodo (10.5281/zenodo.8364925).
References
O’Connell, L. A. & Hofmann, H. A. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol. 519, 3599–3639 (2011).
Wullimann, M. F. & Mueller, T. Teleostean and mammalian forebrains contrasted: evidence from genes to behavior. J. Comp. Neurol. 475, 143–162 (2004).
Yamamoto, N. et al. A new interpretation on the homology of the teleostean telencephalon based on hodology and a new eversion model. Brain Behav. Evol. 69, 96–104 (2007).
Braford, M. R. Jr. Comparative aspects of forebrain organization in the ray-finned fishes: touchstones or not? Brain Behav. Evol. 46, 259–274 (1995).
Northcutt, R. G. Connections of the lateral and medial divisions of the goldfish telencephalic pallium. J. Comp. Neurol. 494, 903–943 (2006).
Nieuwenhuys, R. The development and general morphology of the telencephalon of actinopterygian fishes: synopsis, documentation and commentary. Brain Struct. Funct. 215, 141–157 (2011).
Tosches, M. A. et al. Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science 360, 881–888 (2018).
Colquitt, B. M., Merullo, D. P., Konopka, G., Roberts, T. F. & Brainard, M. S. Cellular transcriptomics reveals evolutionary identities of songbird vocal circuits. Science 371, eabd9704 (2021).
Lust, K. et al. Single-cell analyses of axolotl telencephalon organization, neurogenesis, and regeneration. Science 377, eabp9262 (2022).
Woych, J. et al. Cell-type profiling in salamanders identifies innovations in vertebrate forebrain evolution. Science 377, eabp9186 (2022).
Hain, D. et al. Molecular diversity and evolution of neuron types in the amniote brain. Science 377, eabp8202 (2022).
Pandey, S., Moyer, A. J. & Thyme, S. B. A single-cell transcriptome atlas of the maturing zebrafish telencephalon. Genome Res. 33, 658–671 (2023).
Tarashansky, A. J. et al. Mapping single-cell atlases throughout Metazoa unravels cell type evolution. https://doi.org/10.7554/eLife.66747 (2021).
Styfhals, R. et al. Cell type diversity in a developing octopus brain. Nat. Commun. 13, 7392 (2022).
Johnson, Z. V. et al. Cellular profiling of a recently-evolved social behavior in cichlid fishes. Nat. Commun. 14, 1–19 (2023).
Conte, M. A. et al. Chromosome-scale assemblies reveal the structural evolution of African cichlid genomes. Gigascience 8, giz030 (2019).
Ganz, J. et al. Subdivisions of the adult zebrafish subpallium by molecular marker analysis. J. Comp. Neurol. 520, 633–655 (2012).
Ganz, J. et al. Subdivisions of the adult zebrafish pallium based on molecular marker analysis. F1000Research. 3, 308 (2015).
Maruska, K. P., Butler, J. M., Field, K. E. & Porter, D. T. Localization of glutamatergic, GABAergic, and cholinergic neurons in the brain of the African cichlid fish, Astatotilapia burtoni. J. Comp. Neurol. 525, 610–638 (2017).
Diotel, N. et al. Comprehensive expression map of transcription regulators in the adult zebrafish telencephalon reveals distinct neurogenic niches. J. Comp. Neurol. 523, 1202–1221 (2015).
Pickavance, L. C., Staines W. A., Fryer, J. N. Distributions and colocalization of neuropeptide Y and somatostatin in the goldfish brain. J. Chem. Neuroanat. 5, 221–233 (1992).
Grone, B. P., Butler, J. M., Wayne, C. R. & Maruska, K. P. Expression patterns and evolution of urocortin and corticotropin‐releasing hormone genes in a cichlid fish. J. Comp. Neurol. 529, 2596–2619 (2021).
Tibi, M. et al. A telencephalon cell type atlas for goldfish reveals diversity in the evolution of spatial structure and cell types. Sci. Adv. 9, eadh7693 (2023).
Yamamoto, K., Ruuskanen, J. O., Wullimann, M. F. & Vernier, P. Differential expression of dopaminergic cell markers in the adult zebrafish forebrain. J. Comp. Neurol. 519, 576–598 (2011).
O’Connell, L. A., Fontenot, M. R. & Hofmann, H. A. Characterization of the dopaminergic system in the brain of an African cichlid fish, Astatotilapia burtoni. J. Comp. Neurol. 519, 75–92 (2011).
Adolf, B. et al. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev. Biol. 295, 278–293 (2006).
Gokce, O. et al. Cellular taxonomy of the mouse striatum as revealed by Single-Cell RNA-Seq. Cell Rep. 16, 1126–1137 (2016).
Aoki, T. et al. Imaging of neural ensemble for the retrieval of a learned behavioral program. Neuron 78, 881–894 (2013).
Rink, E. & Wullimann, M. F. The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum). Brain Res. 889, 316–330 (2001).
Northcutt, R. G. Forebrain evolution in bony fishes. Brain Res. Bull. 75, 191–205 (2008).
Mueller, T., Dong, Z., Berberoglu, M. A. & Guo, S. The dorsal pallium in zebrafish, Danio rerio (Cyprinidae, Teleostei). Brain Res. 1381, 95–105 (2011).
Alderman, S. L. & Bernier, N. J. Localization of corticotropin-releasing factor, urotensin I, and CRF-binding protein gene expression in the brain of the zebrafish, Danio rerio. J. Comp. Neurol. 502, 783–793 (2007).
Kleshchevnikov, V. et al. Cell2location maps fine-grained cell types in spatial transcriptomics. Nat. Biotechnol. 40, 661–671 (2022).
Li, B. et al. Benchmarking spatial and single-cell transcriptomics integration methods for transcript distribution prediction and cell type deconvolution. Nat. Methods 19, 662–670 (2022).
Xu, Z. et al. SP8 and SP9 coordinately promote D2-type medium spiny neuron production by activating expression. Development 145, dev165456 (2018).
Volff, J.-N. Genome evolution and biodiversity in teleost fish. Heredity 94, 280–294 (2004).
Zeisel, A. et al. Molecular architecture of the mouse nervous system. Cell 174, 999–1014.e22 (2018).
Ghaddar, B., Lübke, L., Couret, D., Rastegar, S. & Diotel, N. Cellular mechanisms participating in brain repair of adult zebrafish and mammals after injury. Cells 10, 391 (2021).
Grupp, L., Wolburg, H. & Mack, A. F. Astroglial structures in the zebrafish brain. J. Comp. Neurol. 518, 4277–4287 (2010).
März, M. et al. Heterogeneity in progenitor cell subtypes in the ventricular zone of the zebrafish adult telencephalon. Glia 58, 870–888 (2010).
Ming, G.-L. & Song, H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 28, 223–250 (2005).
Hodge, R. D. et al. Tbr2 expression in Cajal-Retzius cells and intermediate neuronal progenitors is required for morphogenesis of the dentate gyrus. J. Neurosci. 33, 4165–4180 (2013).
Butler, A. B. & Hodos, W. Comparative Vertebrate Neuroanatomy: Evolution and Adaptation. (John Wiley & Sons, 2005).
Lein, E. S. et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007).
Ortiz, C. et al. Molecular atlas of the adult mouse brain. Sci. Adv. 6, eabb3446 (2020).
Broglio, C. et al. Hippocampal Pallium and Map-Like Memories through Vertebrate Evolution. J. Behav. Brain Sci. 5, 109–120 (2015).
Broglio, C., Rodríguez, F., Gómez, A., Arias, J. L. & Salas, C. Selective involvement of the goldfish lateral pallium in spatial memory. Behav. Brain Res. 210, 191–201 (2010).
Yamamoto, N. & Ito, H. Visual, lateral line, and auditory ascending pathways to the dorsal telencephalic area through the rostrolateral region of the lateral preglomerular nucleus in cyprinids. J. Comp. Neurol. 508, 615–647 (2008).
Puelles, L., Alonso, A., García-Calero, E. & Martínez-de-la-Torre, M. Concentric ring topology of mammalian cortical sectors and relevance for patterning studies. J. Comp. Neurol. 527, 1731–1752 (2019).
Kyle, A. L. & Peter, R. E. Effects of forebrain lesions on spawning behaviour in the male goldfish. Physiol. Behav. 28, 1103–1109 (1982).
Kondo, Y., Shinoda, A., Yamanouchi, K. & Arai, Y. Role of septum and preoptic area in regulating masculine and feminine sexual behavior in male rats. Horm. Behav. 24, 421–434 (1990).
Goodson, J. L. & Adkins-Regan, E. Effect of intraseptal vasotocin and vasoactive intestinal polypeptide infusions on courtship song and aggression in the male zebra finch (Taeniopygia guttata). J. Neuroendocrinol. 11, 19–25 (1999).
Font, C., Lanuza, E., Martinez-Marcos, A., Hoogland, P. V. & Martinez-Garcia, F. Septal complex of the telencephalon of lizards: III. Efferent connections and general discussion. J. Comp. Neurol. 401, 525–548 (1998).
Yaksi, E., von Saint Paul, F., Niessing, J., Bundschuh, S. T. & Friedrich, R. W. Transformation of odor representations in target areas of the olfactory bulb. Nat. Neurosci. 12, 474–482 (2009).
Blumhagen, F. et al. Neuronal filtering of multiplexed odour representations. Nature 479, 493–498 (2011).
Striedter, G. F. The telencephalon of tetrapods in evolution. Brain Behav. Evol. 49, 179–213 (1997).
Aboitiz, F., Morales, D. & Montiel, J. The evolutionary origin of the mammalian isocortex: towards an integrated developmental and functional approach. Behav. Brain Sci. 26, 535–552 (2003).
Portavella, M., Vargas, J. P., Torres, B. & Salas, C. The effects of telencephalic pallial lesions on spatial, temporal, and emotional learning in goldfish. Brain Res. Bull. 57, 397–399 (2002).
Yamamoto, N. & Ito, H. Fiber connections of the anterior preglomerular nucleus in cyprinids with notes on telencephalic connections of the preglomerular complex. J. Comp. Neurol. 491, 212–233 (2005).
Bienkowski, M. S. et al. Integration of gene expression and brain-wide connectivity reveals the multiscale organization of mouse hippocampal networks. Nat. Neurosci. 21, 1628–1643 (2018).
Striedter, G. F. Evolution of the hippocampus in reptiles and birds. J. Comp. Neurol. 524, 496–517 (2016).
Rodrı́guez, F. et al. Conservation of Spatial Memory Function in the Pallial Forebrain of Reptiles and Ray-Finned Fishes. J. Neurosci. 22, 2894–2903 (2002).
Durán, E., Ocaña, F. M., Broglio, C., Rodríguez, F. & Salas, C. Lateral but not medial telencephalic pallium ablation impairs the use of goldfish spatial allocentric strategies in a ‘hole-board’ task. Behav. Brain Res. 214, 480–487 (2010).
Nakazawa, K., McHugh, T. J., Wilson, M. A. & Tonegawa, S. NMDA receptors, place cells and hippocampal spatial memory. Nat. Rev. Neurosci. 5, 361–372 (2004).
Chiang, M. -C., Huang, A. J.Y., Wintzer M. E., Oshima, T., McHugh, T. A role for CA3 in social recognition memory. Behav. Brain Res. 354, 22–30 (2018).
Preuss, T. M. & Wise, S. P. Evolution of prefrontal cortex. Neuropsychopharmacology 47, 3–19 (2022).
Wyss, J. M. & Van Groen, T. Connections between the retrosplenial cortex and the hippocampal formation in the rat: a review. Hippocampus 2, 1–11 (1992).
Aggleton, J. P., Wright, N. F., Vann, S. D. & Saunders, R. C. Medial temporal lobe projections to the retrosplenial cortex of the macaque monkey. Hippocampus 22, 1883–1900 (2012).
Mitchell, A. S., Czajkowski, R., Zhang, N., Jeffery, K. & Nelson, A. J. D. Retrosplenial cortex and its role in spatial cognition. Brain Neurosci. Adv. 2, 2398212818757098 (2018).
Pattabiraman, K. et al. Transcriptional regulation of enhancers active in protodomains of the developing cerebral cortex. Neuron 82, 989–1003 (2014).
Saunders, A. et al. Molecular diversity and specializations among the cells of the adult mouse brain. Cell 174, 1015–1030.e16 (2018).
Todd, T. P. & Bucci, D. J. Retrosplenial cortex and long-term memory: molecules to behavior. Neural Plast. 2015, 414173 (2015).
Tennant, S. A. et al. Spatial representation by ramping activity of neurons in the retrohippocampal cortex. Curr. Biol. 32, 4451–4464.e7 (2022).
Aboitiz, F. Comparative development of the mammalian isocortex and the reptilian dorsal ventricular ridge. Evolutionary considerations. Cereb. Cortex 9, 783–791 (1999).
Puelles, L. et al. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J. Comp. Neurol. 424, 409–438 (2000).
Desfilis, E., Abellán, A., Sentandreu, V. & Medina, L. Expression of regulatory genes in the embryonic brain of a lizard and implications for understanding pallial organization and evolution. J. Comp. Neurol. 526, 166–202 (2018).
Ramakrishnan Varadarajan, A., Mopuri, R., Streelman, J. T. & McGrath, P. T. Genome-wide protein phylogenies for four African cichlid species. BMC Evol. Biol. 18, 1 (2018).
Kumar, S. & Hedges, S. B. A molecular timescale for vertebrate evolution. Nature 392, 917–920 (1998).
Cárdenas, A. & Borrell, V. Molecular and cellular evolution of corticogenesis in amniotes. Cell. Mol. Life Sci. 77, 1435–1460 (2020).
Acknowledgements
We dedicate this work to Karen Maruska for her groundbreaking research in teleost neuroanatomy and behavioral neuroscience. We thank the Georgia Tech Petit Institute Molecular Evolution Core for their integral roles in sequencing. This work was supported in part by NIH R01GM101095 and R01GM144560 to J.T.S., NIH F32GM128346 to Z.V.J. and Human Frontiers Science Program RGP0052/2019 to J.T.S., and OD P51OD011132 to Emory National Primate Research Center (Z.V.J.).
Author information
Authors and Affiliations
Contributions
B.E.H. and C.M.B. optimized spatial transcriptomics wetlab protocol. Z.V.J. and B.E.H collected samples and B.E.H. performed all downstream wetlab work and library preparation for spatial transcriptomics. The Petit Institute Molecular Evolution Core at GT performed sequencing for spatial transcriptomics. G.W.G. performed all bioinformatics and computational tasks, including: processing reads, quality control, clustering, marker gene analysis, prediction of the anatomical location of cell-types and cross-species gene expression comparisons. B.E.H. reviewed and synthesized literature on teleost neuroanatomy and cell-type biology and performed manual anatomical annotation of spatial transcriptomics spots. B.E.H. and G.W.G. reviewed literature on comparative vertebrate neuroanatomy. G.W.G. designed downstream statistical analyses with feedback from B.E.H., Z.V.J., and J.T.S., B.E.H., and G.W.G. took the lead on writing the manuscript with contributions from Z.V.J. and critical feedback from Z.V.J. and J.T.S., G.W.G. took the lead on designing and creating figures with critical feedback from B.E.H., Z.V.J., and J.T.S. J.T.S. funded spatial transcriptomics experiments.
Corresponding authors
Ethics declarations
Competing interests
These authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Bradley Colquitt, Ayana Rajagopal and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: David Favero and Luke Grinham. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hegarty, B.E., Gruenhagen, G.W., Johnson, Z.V. et al. Spatially resolved cell atlas of the teleost telencephalon and deep homology of the vertebrate forebrain. Commun Biol 7, 612 (2024). https://doi.org/10.1038/s42003-024-06315-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-06315-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.