Abstract
Physical exercise studies are generally underrepresented in young adulthood. Seventeen subjects were randomized into an intervention group (24.2 ± 3.9 years; 3 trainings/week) and 10 subjects into a passive control group (23.7 ± 4.2 years), over a duration of 6 months. Every two months, performance diagnostics, computerized spatial memory tests, and 3 Tesla magnetic resonance imaging were conducted. Here we find that the intervention group, compared to controls, showed increased cardiorespiratory fitness, spatial memory performance and subregional hippocampal volumes over time. Time-by-condition interactions occurred in right cornu ammonis 4 body and (trend only) dentate gyrus, left hippocampal tail and left subiculum. Increases in spatial memory performance correlated with hippocampal body volume changes and, subregionally, with left subicular volume changes. In conclusion, findings support earlier reports of exercise-induced subregional hippocampal volume changes. Such exercise-related plasticity may not only be of interest for young adults with clinical disorders of hippocampal function, but also for sedentary normal cohorts.
Similar content being viewed by others
Introduction
The key role of the human hippocampus for higher mnemonic functions, e.g., episodic and visuospatial memory1, is long-established, as well as contributions of hippocampal dysfunction to memory impairments in various clinical disorders (e.g., dementia, major depression)2,3: accordingly, there is a growing interest in pharmacological and non-pharmacological treatments which could help to improve hippocampal function. Physical activity as a low-cost low-risk intervention provides an attractive option, across various clinical populations and age groups4. Human magnetic resonance imaging (MRI) studies provided firm indications that regular exercise is associated with higher structural integrity of the hippocampus, including meta-analytical evidence4,5,6,7,8, but only few studies were designed as randomized controlled trials (RCTs) comparing physical activity and control interventions9,10,11,12,13,14. A recent meta-analysis on global hippocampal volume concluded that exercise-induced plasticity was evident in older9,11,12, but not young adults6. Meanwhile, RCT studies in young to middle-aged adults are rare, and often used very short training periods of only a few weeks15,16,17,18. Global hippocampal volume measures may also not be sensitive enough in this age range, where neither childhood and adolescent growth nor age-related atrophy drive the developmental trajectory. Therefore, a main goal of this study was to examine whether subregional hippocampal volumes provide more sensitive measures of exercise-induced plasticity in this age range after a longer training duration of 6 months.
Specifically, analyses of total hippocampal volume may miss selective effects in hippocampal subregions. Growing evidence suggests functional gradients along the hippocampal longitudinal axis, as reflected by differential anatomical circuitries of anterior, body, and posterior subregions19,20,21. Some data indicate fitness-related changes preferentially in anterior (head) subregions of the hippocampus9,11,18,22,23. Moreover, the hippocampus proper consists of several subfields with varying cytoarchitecture, including the cornu ammonis areas (CA1/CA2/CA3/CA4), dentate gyrus and subiculum. Here, the dentate gyrus is of special interest: In rodents, physical activity induces hippocampal cell proliferation, neurogenesis, and synaptic plasticity almost exclusively in this area, and this process is also associated with improved spatial memory performance24,25,26, though it may also foster forgetting of preexisting memories27,28. Although difficult to measure in vivo, indirect evidence (e.g., for angiogenesis29) suggests that physical activity also promotes neurogenesis in the human hippocampus30. While cross-sectional studies observed positive correlations between aerobic fitness level and larger CA1 volumes in male adolescents31, and larger volumes for bilateral CA1, CA4, dentate gyrus granule cell and molecular layers, left CA2–3, and the left hippocampal amygdaloid transition area in middle-aged amateur marathon runners32, complementary evidence from RCT studies in young adulthood is limited and mixed: In addition to CA4/dentate gyrus volume increases after a 16-weeks training16 and positive associations between cardiorespiratory fitness improvements and dentate gyrus/CA3 head volume changes after a 12-weeks training23, an earlier study also suggested CA2/3, subiculum and dentate gyrus volume decreases after 6-weeks intense aerobic exercise training18.
Varying effects of physical exercise on hippocampal subfields may also have differential impact on cognitive functions that depend on the hippocampus and surrounding medial temporal regions, including episodic and spatial memory1. During long-term memory consolidation, the hippocampus is thought to bind together afferent item memory (e.g., objects) and context memory (e.g., spatial locations) information from the perirhinal and parahippocampal cortex, respectively, which is relayed via the entorhinal cortex33,34,35. This relational memory is crucial, for instance, for remembering the precise location of objects in space, which requires information about objects to be integrated into a spatial framework. Within the hippocampus, dentate gyrus granule cells support pattern separation by transforming rich cortical inputs via the entorhinal cortex into sparse outputs towards pyramidal cells in CA3, and the hippocampal output region CA136,37,38. Pattern separation (i.e., computational processes that assure that similar memory contents are stored and retrieved as distinct memory representations) is believed to be a hallmark feature of episodic memory, but also spatial mnemonic processing35,37,38,39.
Various behavioral paradigms aim to maximize demand for this kind of mnemonic discrimination, also in exercise-related studies. For example, a cross-sectional study in young adults40 observed that higher cardiorespiratory fitness was linked with better Mnenomic Similarity Task performance: in this object recognition paradigm, participants have to disambiguate repetitions of original stimuli from lure stimuli which look similar, but not identical to the originals41, and better performance is often interpreted as indirect evidence for better dentate gyrus/CA3 function. Cardiorespiratory fitness increases after a 12-weeks training correlated with improved performance in a facial memory paradigm, at least for participants with low baseline fitness23. Meanwhile, some studies cannot replicate such associations42, or only in elderly43. Notably, one cross-sectional study44 found no associations between physical fitness and performance in the original task, but reported a fitness-related attenuation of age-related decline in a virtual 3D navigation task that afforded mnemonic discrimination of overlapping routes.
This could indicate a higher sensitivity of tasks with stronger spatial (instead of purely object-related) demands, which concurs with observations from similar paradigms. A seminal RCT9 observed that exercise-related increases of hippocampal volume in older adults were correlated with improved performance in a delayed matching-to-sample task where participants had to remember the precise spatial location of dot stimuli on a 2D display. Moreover, a cross-sectional study45 in young adults observed that higher cardiorespiratory fitness was associated with smaller placement errors in a paradigm where young participants had to actively reconstruct the 2D spatial arrangement of five abstract shapes that were displayed immediately before, suggesting higher spatial memory precision. Interestingly, this relationship was mediated by fitness-related differences in hippocampal MR viscoelasticity (but not volume) measures, and later work in young adults suggested a more specific relationship between CA3/dentate gyrus viscoelasticity and task performance46. Meanwhile, hippocampal and surrounding parahippocampal areas are involved in 3D spatial navigation47, e.g., in variants of the Morris water maze48, where the position of a hidden location inside a circular arena has to be recollected by triangulating it from the positions of landmarks outside the arena. Preliminary human data using virtual variants of this task suggest that efficient performance is associated with hippocampal volume49,50, and CA1/2 volumes more specifically51,52. Moreover, cross-sectional studies in adolescents observed that higher physical fitness levels are linked with better task performance and higher hippocampal53 and CA1 subfield31 volumes, but found no evidence for a mediation. Therefore, while some cross-sectional data in young adults suggest positive associations between spatial memory performance and physical fitness, whether fitness increases are linked with improved spatial memory performance still needs to be clarified.
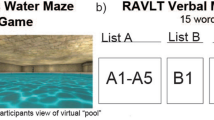
Given the sparsity of data addressing exercise-induced hippocampal subregional plasticity in young adults, the current RCT explored the effects of a 6-months aerobic exercise intervention on hippocampal subregional plasticity and its specific relation to spatial object memory in sedentary young adults. We examined an intervention group (INT) that received regular exercise training and a no-training control group (CON) who both performed MRI and cognitive testing at baseline (T0m), and 2-months (T2m), 4-months (T4m), and 6-months (T6m) follow-ups. We hypothesized to observe differential volume changes in hippocampal subregions, as derived from high-resolution structural MRI, with a special theoretical focus on the dentate gyrus54. Moreover, we tested whether subfield-specific hippocampal changes were linked with training-induced improvements of spatial memory precision in a virtual arena paradigm (spatial object memory task55,56: Fig. 1), hence, supporting subregional hippocampal plasticity as a driver of spatial memory also in young adults.
Participants had to perform a computerized object-location memory task while navigating freely in a circular virtual arena: After an initial learning presentation of an everyday object in a specific spatial position, during subsequent retrieval, participants had to navigate through the arena, and place the cued object as accurately as possible at its original location. Participants received feedback about their accuracy via smiley faces, followed by a re-encoding of the correct object position, to facilitate incremental learning.
Consistent with earlier studies in young adults, no training-related change of global hippocampal volume was observed, but circumscribed changes in specific subregions, suggesting that regular exercise can induce hippocampal plasticity in young sedentary adults. Both groups tended to show improved precision of spatial learning over the 6-months period, but this change was more robust for the INT group. Volume increases of the hippocampal body as well as, at subregional level, the left subiculum volume correlated with better spatial memory over the 6-months period, supporting structural-functional relationships between hippocampal plasticity and spatial memory changes. Yet, these associations were only robust across the whole sample, not within the exercise group only. Therefore, further evidence is needed to confirm the impact of training-induced hippocampal plasticity on complex spatial memory abilities in young adults.
Results
Participants
The cohort was already introduced in previous reports57,58. In total, N = 27 INT and N = 15 CON participants received their allocated treatment. Fourteen participants dropped out within the first 4 months or had to be excluded from final analyses for various reasons (including: loss of interest, time constraints, depressive symptoms, injury due to leisure activities, insufficient training participation due to illness, and non-eligibility for MRI). Further, one subject needed to be excluded from the present analyses due to lack of compliance in the Spatial Object Memory Task. This resulted in a final sample size of N = 27 participants: N = 17 in the INT and N = 10 in the CON group. For T6m follow-ups, MRI was missing in one participant due to new contraindications, and one participant performed neither MRI nor memory examinations, leaving data from N = 25 participants (INT: N = 15, CON: N = 10) for respective analyses. There was no significant difference in drop-out rates between INT and CON (χ2(1,42) = 0.058, p = 0.810), and no systematic difference in background characteristics between study drop-outs and completers (Supplementary Table 1).
Baseline sample characteristics are summarized in Table 1. In general, the sample included young adults who, on average, showed a normal body mass index (BMI) and a fair level of fitness, according to established criteria59 for maximum oxygen consumption relative to body weight (relVO2max). Independent t-tests and χ2 tests revealed no significant baseline group differences for the basic demographic, anthropometric, or psychiatric variables (e.g., age, BMI, relVO2max).
Physiological data—relVO2max
INT participants performed 60.1 ± 11.4 (77 ± 14%) trainings on average out of maximally 78 planned. Due to artifacts, relVO2max values of two INT participants could not be determined, and were therefore excluded from analyses including relVO2max data.
Data showed an increase in relVO2max in INT and a slight decrease in CON participants (Table 2). The Linear Mixed Effect (LME) model for relVO2max revealed a significant effect of time (F(3,64.18) = 5.26, p = 0.003) and time-by-group interaction (F(3,64.18) = 14.02, p < 0.001), however, no group effect (F(1,21.04) = 2.60, p = 0.122). Moreover, a significant effect of sex (F(1,29.99) = 23.22, p < 0.001) indicated higher relVO2max values in males than females. Age had no significant impact (F(1,20.94) = 0.34, p = 0.566).
Post hoc tests within the INT group showed a significant increase in relVO2max from T0m to T2m (t(14) = 5.34, p = 0.001, d = 1.38), T0m to T4m (t(14) = 6.10, p < 0.001, d = 1.58), and T0m to T6m (t(13) = −5.86, p < 0.001, d = −1.57). For CON participants, no significant changes were detected (T0m to T2m: t(6) = −1.45, p = 1.000, d = −0.55; T0m to T4m: t(8) = −1.86, p = 0.601, d = −0.62; T0m to T6m: t(9) = 2.26, p = 0.303, d = 0.71). Comparing INT with CON revealed no significant differences at T0m (t(11.6) = −1.19, p = 0.258, d = −0.56), T2m (t(7.85) = 0.75, p = 0.474, d = 0.42), T4m (t(22) = 0.64, p = 0.528, d = 0.27), or T6m (t(22) = 0.92, p = 0.367, d = 0.38). Comparisons between groups for the deltas were all significant, showing an increase in fitness (relVO2max) in INT and decrease in CON participants (ΔT2T0: t(20) = 4.18, p < 0.001, d = 1.91; ΔT4T0: t(22) = 5.26, p < 0.001, d = 2.22; and ΔT6T0: t(22) = 5.61, p < 0.001, d = 2.52).
Spatial object memory task—mean drop error
The mean drop error (MDE) between the recalled and the actual spatial position of objects within the virtual arena decreased over time (Fig. 2), resulting in a significant main effect of time (F(3,73.10) = 13.58, p < 0.001), while no other significant effects were found (group: F(1,23.06) = 1.69, p = 0.207; time-by-group interaction: F(3,73.10) = 0.99, p = 0.403; age: F(1,23.00) = 0.01, p = 0.934; sex: F(1,23.06) = 0.56, p = 0.463). This indicates that precision of spatial memory performance generally improved across subjects over follow-ups.
Raw values presented with means and standard deviations (as error bars); †† indicates within-group significances at p < 0.01. Data from N = 17 INT and N = 10 CON participants. Abbreviations: CON control group, INT intervention group, T0m baseline measurement, T2m measurement after 2 months, T4m measurement after 4 months, T6m measurement after 6 months.
Exploratory post hoc tests within the INT group showed significant MDE decreases from T0m and T2m (t(16) = −4.83, p = 0.001, d = −1.17), T0m and T4m (t(16) = −4.69, p = 0.001, d = −1.14) and T0m and T6m (t(15) = −4.49, p = 0.003, d = −1.12, whereas CON did not show any significant effects between time points, and smaller effect sizes (T0m to T2m: (t(8) = −2.59, p = 0.193, d = −0.86; T0m and T4m: (t(8) = −2.82, p = 0.136, d = −0.94; T0m and T6m: (t(8) = −1.47, p = 1.000, d = −0.49). Further, there were no significant effects between groups (neither between time points separately (T0m, T2m, T4m, or T6m) nor between the deltas (ΔT6T0, ΔT4T0, and ΔT2T0)).
When we investigated MDE in only the first 20 trials per session (motivated by the idea that these early trials might be more sensitive to physical activity-dependent changes), we obtained similar results as with all 160 trials per session (significant main effect of time: F(3,73.31) = 16.66, p < 0.001; no effect of group: F(1,23.14) = 1.19, p = 0.29; no time-by-group interaction: F(3,73.31) = 0.64, p = 0.59). Secondary analyses showed that participants became faster at completing the 160 trials per session over time (F(3,73.14) = 8.47, p < 0.001) and that INT and CON needed similar amounts of time to complete each session (F(1,23.01) = 3.50, p = 0.07).
Structural data—whole hippocampus
Whole Hippocampus: Only a trend-level main effect of group (F(1,22.00) = 4.1, p = 0.055), but no main effect of time (F(3,73.00) = 0.7, p = 0.533) or time-by-group interaction (F(3,73.00) = 0.1, p = .960) were observed.
Structural data—head/body/tail
For the head region, only a significant main effect of group (F(1,22.00) = 5.5, p = 0.029), but no main effect of time (F(3,73.01) = 0.4, p = 0.764) or time-by-group interaction (F(3,73.01) = 0.9, p = 0.437) were observed. Neither significant main (group: F(1,22.00) = 1.1, p = 0.312; time: F(3,73.00) = 0.5, p = 0.680) nor interaction effects (F(3,73.007) = 1.3, p = 0.275) were observed for the tail region.
Meanwhile, a significant interaction effect emerged in the hippocampal body (F(3,73.00) = 2.89; p = 0.041). No main effects (group: F(1,22.00) = 1.8, p = 0.190; time: F(3,73.0) = 0.5, p = 0.679) were observed.
Post hoc analyses revealed no significant effects between time points within the INT or CON. However, between-group comparisons revealed a significant difference for the change in volume ΔT6T0 (t(23) = 2.93; p = 0.023; d = 1.19), characterized by an increase in volume in the INT and a decrease in the CON (Fig. 3a). No significant differences were observed for ΔT4T0 or ΔT2T0, respectively. Between-group comparisons for each time point separately (T0m, T2m, T4m, or T6m) revealed no significant differences.
a Hippocampal body; b right CA4 body; c right parasubiculum, and d right presubiculum head. Presented are the deltas between T0m and T2m (ΔT2T0), T0m and T4m (ΔT4T0) as well as T0m and T6m (ΔT6T0) for both groups, along with means and standard deviations (as error bars). Data from N = 17 intervention (INT) and N = 10 control (CON) participants. * indicates between-group significances at p < 0.05.
Structural data—hippocampal subfields
The following descriptions focus on hippocampal subfields where significant (or trend-level significant) group × time interactions indicated training intervention-related effects. The complete results of the statistical analyses are listed in Supplementary Table 2.
Right CA4 [body]: A significant interaction effect (F(3,73.00) = 3.42; p = 0.022), but no main effects (group: F(1,22.00) = 0.1, p = 0.830; time: F(3,73.00) = 0.6, p = 0.606) were observed. Post hoc analyses revealed no further effects (Fig. 3b).
Right parasubiculum: A significant interaction effect (F(3,73.01) = 2.99; p = 0.037) and main effect of group (F(1,22.01) = 5,75; p = 0.025) were observed, but no main effect of time (F(3,73.01) = 0.3, p = 0.820). Post hoc analyses showed no further effects (Fig. 3c).
Right presubiculum [head]: A significant interaction (F(3,73.00) = 3.89; p = 0.012) and main effect of group (F(1,22.00) = 5.12; p = 0.033), but no main effect of time (F(3, 73.01) = 1.0, p = 0.391) was observed. Between-group comparisons revealed a significant difference for the change in volume ΔT4T0 (t(25) = −3.23; p = 0.010; d = −1.29) but not for ΔT2T0 or ΔT6T0 (Fig. 3d). Between-group comparisons for each time point separately (T0m, T2m, T4m, or T6m) did not reveal significant differences. Post hoc analyses within groups between time points also did not reveal any further significant effects.
Right granule cell and molecular layer of the dentate gyrus [body]: Analysis revealed a trend for an interaction (F(3,73.00) = 2.41; p = 0.074), but no main effects (group: F(1,21.997) = 0.3, p = 0.608; time: F(3,73.003) = 0.5, p = 0.693). Post hoc analyses revealed no further effects (Fig. 4a).
a Right granule cell and molecular layer of the dentate gyrus body; b left hippocampal tail; c left molecular layer of the hippocampus body, and d left subiculum body. Presented are the deltas between T0m and T2m (ΔT2T0), T0m and T4m (ΔT4T0) as well as T0m and T6m (ΔT6T0) for both groups, along with means and standard deviations (as error bars). ** indicates between-group significances at p < 0.01, † indicates within-group significance at p < 0.05. Data from N = 17 intervention (INT) and N = 10 control (CON) participants.
Left hippocampal tail: Analysis revealed a trend for an interaction (F(3,73.01) = 2.19; p = 0.097), but no main effects (group: F(1,22.01) = 0.9, p = 0.366; time: F(3,73.01) = 0.0, p = 0.996). Post hoc analyses revealed no further effects (Fig. 4b).
Left molecular layer [body]: Analysis revealed a trend for an interaction (F(3,73.01) = 2.39; p = 0.075), but no main effects (group: F(1,22.00) = 1.4, p = 0.245; time: F(3,73.008) = 0.4, p = 0.782). Post hoc analyses within group revealed a trend increase between the time points T0m and T6m in the INT (t(14) = −2.44; p = 0.086; d = −0.63) and a significant decrease between T0m and T6m in the CON group (t(9) = 3.19; p = 0.033; d = −1.01) (Fig. 4c). Importantly, between-group comparisons showed a significant differential change in volume (increase in INT and decrease in CON) for ΔT6T0 (t(23) = 3.54; p = 0.005; d = 1.45) (Fig. 4c). No significant differences were observed for ΔT2T0 or ΔT4T0. Between-group comparisons for each time point separately (T0m, T2m, T4m, or T6m) did not reveal any significant differences.
Left subiculum [body]: Analysis revealed a trend for the interaction (F(3,73.01) = 2.41; p = 0.073), but no further main effects (group: F(1,22.003) = 2.1, p = 0.164; time: F(3,73.010) = 0.3, p = 0.832). Post hoc analyses within group revealed a significant increase between T0m and T4m in INT participants (t(16) = −2.78; p = 0.041; d = −0.67), but no effects between T0m and Tm2 or T0m and T6m (Fig. 4d). Within CON group, no significant effects were observed. Between-group comparisons for changes in volumes (ΔT2T0, ΔT4T0, and ΔT6T0) also revealed no significant effects. There was only a trend for change in volume between groups (increase in INT and decrease in CON) for ΔT6T0 (t(23) = 2.32; p = 0.089; d = 0.95). Between-group comparisons for each time point separately (T0m, T2m, T4m, or T6m) did not reveal any significant differences.
Entorhinal cortex: An exploratory analysis for the left and right entorhinal cortex found no evidence for main effects of group or time, or time-by-group interactions (Supplementary Table 2).
Correlations between hippocampal volumes, fitness parameters, and spatial memory performance
Analyses of both groups combined revealed a significant negative correlation between the MDE and left subiculum body volume (r = −0.350, p = 0.047), showing that a decrease in MDE goes along with an increase in left subiculum body volume (Fig. 5a). While subgroup analyses showed similar small to moderate negative correlations, these associations were weaker, and missed significance in both groups (INT: r = −0.242, p = 0.192; CON: r = −0.322, p = 0.199). Further, an analogous trend was observed for the (negative) correlation between the MDE and the hippocampal body volume (r = −0.290, p = 0.085) (Fig. 5b). Subgroup analyses showed smaller correlations, which again failed to reach significance (INT: r = −0.187, p = 0.252; CON: r = −0.18, p = 0.322).
Correlations between the changes in Mean Drop Error from baseline to 6-months follow-up (MDE(ΔT6T0)) and the complementary volume changes of the a) left subiculum body (ΔT6T0) (r = −0.35, significant) and b) hippocampal body (ΔT6T0) (r = −0.29, trend only). The scatterplots present correlations across groups, i.e., for intervention (INT) and control (CON) participants combined. Data from N = 15 INT and N = 9 CON participants.
Meanwhile, we found no evidence for significant positive correlations between relVO2max and volume increases in global hippocampal, subregional or subfield analyses.
Discussion
This exploratory randomized aerobic exercise intervention over a 6-month period in untrained young adults extends previous evidence that regular physical activity induces changes in fitness, subregional hippocampal plasticity, and spatial learning in humans. As expected, significant increases in fitness were observed in the INT, but not in the CON group, where a slight decrease was seen. Overall improvements in spatial learning in a virtual arena paradigm were found, as indexed by reductions in MDE compared to baseline, and were more robust for the INT than in the CON group (though without significant differences when comparing both groups directly). Consistent with earlier studies in young adults, no evidence was found for training-related changes of global hippocampal volume, as indicated by condition-by-time interactions, but more circumscribed changes in specific subregions. There was a significant interaction for the hippocampal body region, reflected by a trend increase in volume in the INT and decrease in the CON group. At the subfield level, an analogous pattern was observed for the right CA4 body. Similar interactions were found in body regions of the granule cell and molecular layer of the dentate gyrus, left molecular layer, left subiculum, and left hippocampal tail, albeit at trend level only. On the other hand, inverse significant interactions characterized by a trend for volume decreases in the INT and increases in the CON group, respectively, emerged in the right parasubiculum and anterior right presubiculum. Interestingly, correlational analysis focusing on the differences (deltas) between T0m and T6m revealed that MDE reductions, indexing spatial memory improvements over time, correlated with global hippocampal body volume increases as well as with, at the subregional level, volume increases in the left subiculum.
Fitness improvements, demonstrated as relVO2max increases, were found in INT participants only, largely confirming the results of other randomized training interventions in similar age groups of 2-month60,61 to 6-month62 durations, or meta-analyses of exercise interventions in young adults aged <40 years63. Findings indicate that the 6-month aerobic exercise intervention was effective in improving fitness levels, hence, rendering the intervention suitable for subsequent differential statistical analyses of imaging and behavioral changes between time points and groups, as well as their inter-relationships in correlational analyses.
Turning to the structural MRI analyses, whole hippocampal volume showed no significant training-related changes, consistent with previous studies16. A meta-analysis from N = 23 interventional studies reported a positive effect of exercise on total hippocampal volume in older samples (>65 years), and preferentially in interventions that lasted >24 weeks and <150 min exercise/ week6. Hippocampal volume in elderly may be especially sensitive to the positive influence of physical activity because it counteracts ongoing aging-related changes, i.e., serves hippocampal maintenance10,64. This anticipated lack of global volume changes in our young adult cohort supported the subregional analysis approach. In fact, we did find evidence for differential changes in the hippocampal body region: Between-group comparisons revealed a significant difference in the pre- (T0) to post- (T6) intervention changes that was driven by volume increase in INT and decrease in CON participants. This regional pattern is interesting, given that previous studies mainly suggested exercise-induced volume changes in anterior (head) subregions: While age-related differences could explain the variation from findings in elderly cohorts9,11, an RCT in young- to middle-aged adults22 also found a positive effect of a 6-weeks exercise training on anterior (but not whole) hippocampal volume that returned to baseline after a 6-weeks follow-up without training. Another study23 indicated positive effects of a 12-week exercise intervention in the left anterior hippocampus. Meanwhile, there is a contrary finding from a voxel-based analysis (i.e., volume decrease in the anterior right hippocampus of the training intervention) after a 6-weeks exercise program18. Based on the limited available evidence, it is difficult to evaluate how much the observed heterogeneity relates to methodological factors (e.g., training content and duration).
Considering specific hippocampal subfields, significant training-related changes were detected in the right CA4 body. Anatomically, the multiform layer of the dentate gyrus merges without sharp demarcation into the CA4 region (hilus) of the cornu ammonis65 and other human imaging studies have thus reported on one merged dentate & CA4 region (dentate gyrus/CA4)66,67. Using slightly different segmentation schemes, recent studies including young adults provide further evidence for training-induced volume increases in dentate gyrus/CA323 and CA4/dentate gyrus subregions16, although preferentially in the left hemisphere. Complementary observations from cross-sectional studies also indicate positive associations of CA4 volume with physical fitness levels32. In the rodent hippocampus, physical activity has been shown to preferentially facilitate neurogenesis in the dentate gyrus, and its granule cell and molecular layer more specifically24,25,26,54,68,69. In this line, Pereira et al.29 compared interventional observations in rodents and young to middle-aged humans: aerobic exercise was found to have a primary effect on cerebral blood volume, an imaging correlate of neurogenesis, in the dentate gyrus of both, mice and men. However, this human 12-week exercise intervention included only a small sample of 11 subjects and had no control group29. While the present study found no effects in the dentate gyrus proper, and only a trend-level interaction for the body region of the granule cell and molecular layer of the right dentate gyrus, the latter finding is difficult to interpret, since it was mainly characterized by a trend for volume decrease in the CON group, with no systematic change in the INT group.
Although the animal literature makes the dentate gyrus a promising candidate for exercise-induced plasticity also in the human hippocampus, work by Sorrells et al.70 provided evidence that the subgranular zone of the dentate gyrus, contrary to what is well-established in the adult mammalian brain, does not seem to be the major site of neurogenesis in human adults. Interestingly, Nogueira et al.71 discuss that neurogenesis in the adult human hippocampal formation may shift from the subgranular zone to the subiculum and the molecular layers of the hippocampus proper72. Therefore, it is interesting that the current study found preliminary evidence for exercise-induced volume increases in the left subiculum and the molecular layer. While not regularly observed in exercise-related studies, there is some cross-sectional evidence for a positive relationship between cardiorespiratory fitness and subicular volume in elderly women73.
The present analyses also found trend-level evidence for time-by-group interactions in additional subfields, including the left hippocampal tail. Meanwhile, there were also two subfields, the right parasubiculum and right head of the presubiculum, which showed a reversed trend, i.e., smaller volumes for the INT group over time. Given that there is no previous literature showing exercise-induced volume changes in these subregions, these findings are difficult to interpret. In general, it was already noted that some studies in young adults made counterintuitive observations of regional volume reductions after exercise interventions18 (see also14) which could indicate more complex patterns of subregional structural plasticity that need further systematic exploration, although technical factors may also play a role. These aspects also need to be taken into account when considering the observation that the time-by-group interactions were often driven by both an increasing trend in the training group and a decreasing trend in the control group. Similar patterns were also observed in an earlier study16. Although there is preliminary evidence from developmental studies that hippocampal74, and also hippocampal subfield volumes75,76 may already start to decline in the early 20 s (or even earlier), the limited observation duration (6 months) seems too short to detect such changes reliably on the intraindividual level.
The behavioral outcome of this study, namely, the reduction in MDE as an indicator of improved spatial memory accuracy in the virtual arena was generally shown to improve over time: Exploratory post hoc analyses showed that they were only significant in the INT (reduction compared to baseline), but not in the CON group, even though nominal effect sizes showed that some improvement was also present in this smaller group. Thus, INT participants became significantly more accurate over time in an established spatial learning paradigm55,56,77,78. While the influence of practice effects cannot be discarded, as also the CON showed some (nonsignificant) improvement, the effect sizes for the INT were higher, suggesting at least subtle improvements due to exercise. An effect of regular physical activity on spatial learning is supported by previous cross-sectional observations that higher cardiorespiratory fitness was positively linked with mnemonic precision in 2D45 and 3D spatial memory task contexts44,53. On the other hand, a recent longitudinal study79 involving 20 young adults found no direct evidence for improved spatial learning after a 12 weeks mild-intensity aerobic exercise intervention: The intervention neither affected relVO2max, nor spatial learning in virtual maze tasks. This negative behavioral outcome, in comparison to our study, might be related to the shorter length and lower intensity of the exercise intervention79.
As it comes to possible morphological underpinnings of improved spatial learning after regular exercise training, the reduction in MDE over time was correlated across groups with volume increases in two of the subregions that showed training-related effects, the body part of the left subiculum and, marginally, the hippocampal body (which included the respective subicular subfield). Subgroup analyses showed similar negative trends in both groups, although these effects failed to reach significance: In general, given that the correlation patterns were not specific for the INT group, one has to be careful with inferring a causal relationship between training, structural changes, and spatial memory performance. While hippocampal specialization has long been discussed in the context of an anterior–posterior dichotomy, recent data suggest that at least a tripartite functional scheme including the body region may be more appropriate20. There is some evidence that the hippocampal body region is implicated in spatial memory, and that relationships between hippocampal body volume and visuospatial memory performance are not restricted to elderly or clinical populations, but can also be observed in young and middle-aged adults21. A specific role of the subiculum in spatial learning is suggested80 by lesion studies81,82 and cell recordings showing that subicular cells code the animal’s direction during open field exploration, which is not the case for hippocampal place cells83,84. However, subicular volume was not regularly associated with task performance in this sort of paradigms in human studies, or visuospatial memory more generally21. In fact, previous human studies utilizing tasks for spatial mnemonic discrimination, or spatial navigation, tended to draw relationships with CA131,51,52 or CA3/dentate gyrus integrity46. Meanwhile, if changes in cardiorespiratory fitness should modulate subicular volume, such complex task modalities should provide the most sensitive behavioral measure, also given earlier findings in elderly that cardiorespiratory fitness was linked with better performance in a spatial route disambiguation task44,73. Although the present and previous studies do not yet provide a coherent picture, they provide evidence that associations between hippocampal structural plasticity and changes in hippocampus-dependent memory function cannot only be observed in aging or early development, but across the whole lifespan.
While strengths include the randomized study design in combination with multiple longitudinal assessments of hippocampal volume changes (instead of simple pre-post comparisons) and behavioral assessment of a complex spatial memory task, this study is not without limitations. Despite some evidence in favor of relative volume increases for the INT group, no linear correlations between fitness gains and volume changes were observed, which would have provided the strongest support for exercise-related changes in hippocampal structure. The hippocampal subfield atlas introduced in FreeSurfer 685 was utilized, which means that the subfield definitions are not completely consistent with those in some abovementioned studies (which used earlier FreeSurfer implementations, or other segmentation schemes). There have been general reservations regarding the reliability of such fully automated segmentation pipelines, although studies using the most recent implementations provide more favorable results86. The study was conducted in a mainly European sample, which may limit the generalizability to other ethnic backgrounds. The major limitation that we have to acknowledge is the small number in particular of CON participants finalizing the study which negatively impacted the statistical power of the overall study design, and minimized chances to find significant changes. This limitation is partially reduced by the longitudinal follow-up with three consecutive MRI sessions which confirmed stable results over time in the CON, and very different evolution in the INT. We cannot exclude the risk of false positive findings due to the multitude of performed tests. While analyses for the basic background characteristics indicate no systematic drop-out of participants, we cannot completely exclude selective attrition effects that may overestimate the observed effects, and limit generalizability. As several drop-outs occurred early, it was not feasible to test this directly in a systematic intention-to-treat analysis, due to missing follow-ups. We decided to use a passive control condition, as was discussed in further detail in previous resting-state fMRI57 and perfusion studies58 from this cohort: While advantages and disadvantages of different types of control conditions have been discussed in the literature, potential bias should not have a major impact on structural data, as in the current study.
In conclusion, this RCT with multiple imaging time-points over a 6-month period in initially untrained young adults identified differential changes in INT, when compared to CON participants. Findings indicate that hippocampal plasticity in young sedentary adults can be induced by regular physical activity, and while there is no evidence for global changes in hippocampal volume (as sometimes observed in elderly), there were regional changes that were partially consistent with animal findings. Meanwhile, spatial learning was particularly linked with local volume increases of the hippocampal body and the subiculum. Because studies of physical activity in young adulthood are generally underrepresented, further interventions should be conducted to elucidate the changes in brain plasticity at this early stage of life that may maximally mediate the long-term benefits of physical activity. Future studies will need larger samples of individuals, ideally over the course of a longer-term intervention (>6 months), to examine the stability of the effects of regular exercise on hippocampal integrity, and incorporate behavioral measures (including functional MRI paradigms) of complex spatial learning abilities. If hippocampal plasticity is not only limited to elderly (serving maintenance against aging-related or neurodegenerative decline), but also present in young adults, this would not only be interesting for clinical populations in this age range but probably also for sedentary individuals who may not only profit from the general health benefits of cardiovascular fitness but also from improved cognitive functioning, especially in hippocampus-related complex memory domains.
Methods
The present sub-study is part of the “RUNSTUD” study, from which data on other research questions have already been published57,58,87. RUNSTUD was a randomized 6-month exercise intervention study in young, healthy, and sedentary subjects which included the acquisition of performance diagnostics, MRI, neuropsychological tests, pain measurements, and blood sampling for epigenetics. The study is registered in the German Register of Clinical Studies (DRKS00021460). A detailed description of the study design was published in Maurer et al.57.
Participants
Healthy sedentary women and men, aged 18–35 years, with no prior history as competitive athletes and/or regular physical exercise training in the last 2 years preceding the study were recruited through social media and via flyers at the local university. Exclusion criteria were psychiatric, neurologic, or cardiovascular diseases (current or in the past). Before each MRI session, participants were informed and screened for contraindications. Female participants were additionally tested for pregnancy.
Written informed consent was obtained after participants were notified about the study purpose, all tests, and potential risks. The study was approved by the Ethics Committee at the Medical Faculty of the Rheinische Friedrich–Wilhelms-Universität Bonn (no. 370/15) and conducted according to national legislation and the Declaration of Helsinki. All ethical regulations relevant to human research participants were followed.
Experimental procedure
At study inclusion, demographic characteristics (age, educational level, and handedness) and psychiatric questionnaires (such as the Beck Depression Inventory (BDI-I88), the trait-anxiety scale of the State-Trait-Anxiety Inventory (STAI89), and the Mini International Neuropsychiatric Interview (M.I.N.I., German version 5.0.090)) were obtained from all subjects. In addition, a questionnaire about substance consumption and the Fagerström Test for Nicotine Dependence (FTND91) were gathered. To rule out serious pre-existing health conditions, a medical health check was performed subsequent to performance diagnostics and trainings. Thereafter, participants were randomized in one of two groups: the INT group or the passive CON group. The sequential list randomization method was used to assign subjects in a 2:1 ratio (INT:CON) since higher dropout rates in the INT group were expected.
At baseline (T0m) and every 2 months during the study (after two (T2m), four (T4m), and 6 months (T6m)), subjects underwent performance diagnostics (graded exercise test) to determine individual fitness, a 3 Tesla MRI examination, and a spatial object memory task. The INT group received extensive interval training (3 training sessions per week of 25–45 min duration) tailored to their individual fitness levels. The CON group was told not to deviate from their routines. For further details on performance diagnostics and exercise intervention see Maurer et al.57. As a fitness outcome, we used the maximum oxygen consumption relative to body weight (relVO2max), which was defined as the highest 30 s moving average of VO2 divided by body mass (ml min−1 kg−1).
Spatial object memory task
Participants performed a computerized spatial object memory task, which was adapted from multiple previous studies55,56,77,78. These previous studies established that this task has a high validity to investigate spatial memory in humans and that it resembles various spatial navigation and spatial memory tasks in rodents such as the Morris Water Maze task92. They demonstrated, amongst others, that the human entorhinal cortex shows a macroscopic functional magnetic resonance imaging (fMRI) signal that may result from the activity of grid cells93 and that single neurons in the human medial temporal lobe encode directions and distances during virtual spatial navigation55. Previous studies with similar virtual reality paradigms showed that they correlate strongly with real-world spatial memory performance, further supporting the validity of this task for assessing spatial memory94.
The paradigm was developed using Unreal Engine 2 (Epic Games, Cary, NC, USA), and is illustrated in Fig. 1. Participants were located in a virtual arena that comprised a grassy plain which was surrounded by a cylindrical cliff. The background scenery included a large and a small mountain, clouds, and the sun: These distal landmarks were rendered at infinity and remained stationary throughout the task. There were no intramaze landmarks. Subjects were able to navigate through the virtual arena by using the arrow keys of a keyboard.
At the beginning of the task, subjects learned the locations of eight everyday objects (randomly drawn from a total number of 12 potential objects: eggplant, baby bottle, briefcase, globe, bucket, rubber duck, barrel, stapler, agenda, vase, alarm clock, basketball) by collecting them from different locations in the arena (“initial learning phase”; total duration 2–3 min). Afterwards, subjects completed 160 trials (20 trials per object in random order), each starting with an inter-trial interval of 3–5 s duration (uniformly distributed). Then, one of the 8 objects (“cue”; duration of 2 s) was presented at the top of the screen. During the subsequent retrieval period (“retrieval”; self-paced), subjects navigated to the remembered object location and indicated their arrival with a button press. Next, subjects received feedback on the accuracy of their response using one of 5 different emoticons (“feedback”; happy to sad; duration of 1.5 s). The retrieved object then appeared in its correct location, and subjects had to navigate there to collect it, to further improve their associative object-location memories (“re-encoding”; self-paced). Because the task was self-paced, total duration depended on how fast the subjects completed the 160 trials.
Every 0.1 s, the participant’s position in the arena was logged, which allowed to extract movement periods, speed, and direction. Response accuracy was measured as the Euclidean distance between the response location and the correct location (“drop error”). To quantify subjects’ spatial memory performance, a mean drop error (MDE) over all 160 trials was calculated by averaging across all raw drop-error values (without any further transformations), which served as a dependent variable for statistical analysis. The lower the MDE, the better the subjects’ spatial memory performance.
To assess the reliability of the computerized spatial object memory task, we estimated the MDE for each session and computed Cronbach’s alpha across different sessions. We found that Cronbach’s alpha was 0.958, indicating excellent reliability of assessing spatial memory performance with this task.
MRI acquisition
MRI scans were performed on a 3 Tesla Siemens Magnetom Skyra System with a 32-channel head coil. Anatomical T1 weighted (T1w) images were obtained with an in-house developed MP-RAGE sequence with 1 × 3z1 CAIPIRINHA and elliptical sampling95: sagittal slice orientation, voxel size = 1 × 1 × 1 mm, field-of-view = 192 × 192 × 144 mm, TR = 2.5 s, TI = 1.1 s, TE = 5 ms, flip angle = 7°, total scan duration: 2 min 53 s.
Hippocampus segmentation
The structural MR images were automatically segmented using the longitudinal stream96 provided in FreeSurfer 6.0 (http://surfer.nmr.mgh.harvard.edu/). By means of robust, inverse consistent registration, this workflow generates an unbiased within-subject template space and image97. Based on common information from this within-subject template, several of the processing steps of the default FreeSurfer pipeline98 for cross-sectional data (e.g., skull stripping, Talairach transformation, atlas registration, spherical surface mapping and parcellation) are initialized, which increases reliability and statistical power of the longitudinal stream significantly96.
Hippocampal subfield segmentation utilized the hippocampal subfields and amygdala nuclei atlas85 in FreeSurfer 7.0. Technical details regarding the procedure and segmented hippocampal subfield can be found online (https://surfer.nmr.mgh.harvard.edu/fswiki/HippocampalSubfieldsAndNucleiOfAmygdala). Volume measurements were obtained from 12 subfields, each for the left and right hemispheres, and some of them were further divided into head and body divisions. Subfields were: (i) parasubiculum, (ii) presubiculum [head and body], (iii) subiculum [head and body], (iv) CA1 [head and body], (v) CA3 [head and body] which also includes CA2, (vi) CA4 [head and body], (vii) granule cell and molecular layer of the dentate gyrus [granule cells-molecular layer-dentate gyrus, head and body], (viii) molecular layer [head and body], (ix) hippocampal amygdaloid transition area, (x) fimbria, (xi) hippocampal tail, and (xii) hippocampal fissure. Segmentation resulted in 19 subdivisions (including head/body divisions) and a total of 38 subdivisions across hemispheres. For comparison with previous investigations, the subfield volumes were also combined to calculate a whole hippocampus measure, and, following suggestions that exercise effects may vary along the longitudinal hippocampal axis9,11,18,22,23, created composites for head (including left and right parasubiculum, presubiculum [head], subiculum [head], CA1 [head], CA3 [head], CA4 [head], granule cell and molecular layer of dentate gyrus [head], molecular layer of the hippocampus [head], and hippocampal amygdaloid transition area), body (including left and right presubiculum [body], subiculum [body], CA1 [body], CA3 [body], CA4 [body], granule cell and molecular layer of dentate gyrus [body], molecular layer of the hippocampus [body], and fimbria) and tail (left and right hippocampal tail).
Due to previous studies with the behavioral task that indicated a central role of the entorhinal cortex56, we also analyzed the left and right entorhinal volumes for exploratory purposes.
Finally, the pipeline included the estimation of total intracranial volume (eTIV) for each subject99 as a covariate for later statistical analyses.
The ENIGMA quality control protocol (http://enigma.ini.usc.edu/) was used for visual quality control of the automatic hippocampus segmentation100.
Statistics and reproducibility
Statistical analyses were conducted using IBM SPSS Statistics version 27 (IBM Corp., Armonk, New York). To test for baseline differences between groups, participants´ characteristics were analyzed using independent t-tests for interval data, and χ2 tests for frequency data.
Analyses of longitudinal physiological (relVO2max), behavioral (Spatial Object Memory Task), and structural MRI data (hippocampal subfields) were performed using linear mixed effects (LME) models. Covariates age and sex (as fixed effects) and random intercepts were added to account for random individual level effects. Additionally, eTIV was added as a covariate of no interest in structural data analyses to account for variations in individual brain size.
Assumptions for applying the linear mixed effects model (linearity and normality) were tested by performing Shapiro-Wilk tests and visual checks of the Q–Q plots of the model residuals for normality and by visually checking the plots between model residuals vs the predictor for linearity. Further, we checked for outliers by checking the distribution of residuals. All hippocampal subfield and MDE residuals showed approximately normal distributions (except for the hippocampal subfield “right CA3 [body]”). Further, all plots of the hippocampal subfield and the MDE predictors and their LME model residuals looked random, in line with the assumption of linearity. The investigation of outliers revealed no extreme outliers. Therefore, we included all subjects’ data for final analysis.
In case of significant effects (time, group, or time-by-group interaction), post hoc tests between time points within each group were conducted. Between-group comparisons were performed for each time point separately but also for the change in volume over time (delta T2m minus T0m (ΔT2T0), delta T4m minus T0m (ΔT4T0), and delta T6m minus T0m (ΔT6T0)). p-values were adjusted using Bonferroni correction. Results of post hoc tests were considered significant when p < 0.05 and are reported with Cohen’s d effect size101.
The subfields hippocampal amygdaloid transition area and hippocampal fissure were not of primary interest in this study and therefore are reported in Supplementary Note 1 and Supplementary Fig. 1.
Correlation analyses
Correlation analyses were performed between the change in relVO2max and behavioral changes in the Spatial Object Memory Task (MDE) and changes in the hippocampal subfield volumes. Therefore, differences between T0m and T6m were calculated by subtracting baseline values from the follow-up assessment. Due to the small sample sizes, we only correlated the values of both groups (INT and CON) together. Positive associations between increased subregional volume and increased relVO2max or decreased MDE, respectively, were considered significant (p < 0.05, one-tailed).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to containing information that could compromise research participant privacy/consent. Data can only be made available by the corresponding author (H.B.) after contacting participating volunteers and obtaining their consent to submit data in pseudonymized form (as per ethics’ approval). Depending on the decision of the volunteers, this may result in smaller samples. The data used to make the plots in Figs. 2–5 can be found in Supplementary Data 1.
Code availability
The spatial object memory task was adapted from previous studies (Doeller et al., 2008: https://doi.org/10.1073/pnas.0711433105; Kunz et al, 2015: https://doi.org/10.1126/science.aac8128), and developed using Unreal Engine 2 (Epic Games, Cary, NC, USA). Custom MATLAB code generated during this study for behavioral data analysis of the Spatial Object Memory Task is available at https://github.com/NeuroLuke/BoeckerCommunicationsBiology2024. Structural MRI data were preprocessed using the FreeSurfer 6.0 and 7.0 standard workflows for longitudinal and hippocampal subfield segmentation, respectively. Demographic, behavioral, physiological and volumetric data were analyzed using default procedures provided in SPSS 27.0 (IBM Corp, Armonk, NY).
References
Eichenbaum, H. & Cohen, NealJ. Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron 83, 764–770 (2014).
Dillon, D. G. & Pizzagalli, D. A. Mechanisms of memory disruption in depression. Trends Neurosci. 41, 137–149 (2018).
Nathan, P. J. et al. Association between CSF biomarkers, hippocampal volume and cognitive function in patients with amnestic mild cognitive impairment (MCI). Neurobiol. Aging 53, 1–10 (2017).
Aghjayan, S. L. et al. Aerobic exercise, cardiorespiratory fitness, and the human hippocampus. Hippocampus 31, 817–844 (2021).
Li, M. Y. et al. The effects of aerobic exercise on the structure and function of DMN-related brain regions: a systematic review. Int. J. Neurosci. 127, 634–649 (2017).
Wilckens, K. A. et al. Exercise interventions preserve hippocampal volume: a meta-analysis. Hippocampus 31, 335–347 (2021).
Ji, L., Steffens, D. C. & Wang, L. Effects of physical exercise on the aging brain across imaging modalities: a meta-analysis of neuroimaging studies in randomized controlled trials. Int. J. Geriatr. Psychiatry 36, 1148–1157 (2021).
Erickson, K. I., Leckie, R. L. & Weinstein, A. M. Physical activity, fitness, and gray matter volume. Neurobiol. Aging 35, S20–S28 (2014).
Erickson, K. I. et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl Acad. Sci. USA 108, 3017–3022 (2011).
Demnitz, N. et al. Hippocampal maintenance after a 12-month physical activity intervention in older adults: the REACT MRI study. Neuroimage Clin. 35, 102762 (2022).
Maass, A. et al. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol. Psychiatry 20, 585–593 (2015).
Rosano, C. et al. Hippocampal response to a 24-month physical activity intervention in sedentary older adults. Am. J. Geriatr. Psychiatry 25, 209–217 (2017).
Jonasson, L. S. et al. Aerobic exercise intervention, cognitive performance, and brain structure: results from the Physical Influences on Brain in Aging (PHIBRA) study. Front. Aging Neurosci. 8, 336 (2016).
Kaiser, A. et al. A randomized controlled trial on the effects of a 12-week high- vs. low-intensity exercise intervention on hippocampal structure and function in healthy, young adults. Front. Psychiatry 12, 780095 (2021).
Woost, L. et al. Physical exercise and spatial training: a longitudinal study of effects on cognition, growth factors, and hippocampal plasticity. Sci. Rep. 8, 4239 (2018).
Frodl, T. et al. Aerobic exercise increases hippocampal subfield volumes in younger adults and prevents volume decline in the elderly. Brain Imaging Behav. 14, 1577–1587 (2020).
Parker, B. A. et al. Effect of exercise training on hippocampal volume in humans: a pilot study. Res. Q. Exerc. Sport 82, 585–591 (2011).
Wagner, G. et al. Hippocampal structure, metabolism, and inflammatory response after a 6-week intense aerobic exercise in healthy young adults: a controlled trial. J. Cereb. Blood Flow. Metab. 35, 1570–1578 (2015).
Duvernoy, H. M., Cattin, F. & Risold, P.-Y. The Human Hippocampus. (Springer Berlin, Heidelberg, 2013).
Strange, B. A., Witter, M. P., Lein, E. S. & Moser, E. I. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 15, 655–669 (2014).
Travis, S. G. et al. High field structural MRI reveals specific episodic memory correlates in the subfields of the hippocampus. Neuropsychologia 53, 233–245 (2014).
Thomas, A. G. et al. Multi-modal characterization of rapid anterior hippocampal volume increase associated with aerobic exercise. Neuroimage 131, 162–170 (2016).
Nauer, R. K., Dunne, M. F., Stern, C. E., Storer, T. W. & Schon, K. Improving fitness increases dentate gyrus/CA3 volume in the hippocampal head and enhances memory in young adults. Hippocampus 30, 488–504 (2020).
Lieberwirth, C., Pan, Y., Liu, Y., Zhang, Z. & Wang, Z. Hippocampal adult neurogenesis: its regulation and potential role in spatial learning and memory. Brain Res. 1644, 127–140 (2016).
Marlatt, M. W., Potter, M. C., Lucassen, P. J. & van Praag, H. Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Dev. Neurobiol. 72, 943–952 (2012).
van Praag, H., Shubert, T., Zhao, C. & Gage, F. H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 25, 8680–8685 (2005).
Akers, K. G. et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 344, 598–602 (2014).
Epp, J. R., Silva Mera, R., Kohler, S., Josselyn, S. A. & Frankland, P. W. Neurogenesis-mediated forgetting minimizes proactive interference. Nat. Commun. 7, 10838 (2016).
Pereira, A. C. et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl Acad. Sci. USA 104, 5638–5643 (2007).
Ho, N. F., Hooker, J. M., Sahay, A., Holt, D. J. & Roffman, J. L. In vivo imaging of adult human hippocampal neurogenesis: progress, pitfalls and promise. Mol. Psychiatry 18, 404–416 (2013).
Prathap, S., Nagel, B. J. & Herting, M. M. Understanding the role of aerobic fitness, spatial learning, and hippocampal subfields in adolescent males. Sci. Rep. 11, 9311 (2021).
Ao, Y.-W. et al. Hippocampal subfield volumes in amateur marathon runners. Med. Sci. Sports Exerc. 55, 1208–1217 (2023).
Diana, R. A., Yonelinas, A. P. & Ranganath, C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn. Sci. 11, 379–386 (2007).
Eichenbaum, H. Memory: organization and control. Annu. Rev. Psychol. 68, 19–45 (2017).
Ekstrom, A. D. & Yonelinas, A. P. Precision, binding, and the hippocampus: precisely what are we talking about? Neuropsychologia 138, 107341 (2020).
Hainmueller, T. & Bartos, M. Parallel emergence of stable and dynamic memory engrams in the hippocampus. Nature 558, 292–296 (2018).
Garthe, A. & Kempermann, G. An old test for new neurons: refining the Morris water maze to study the functional relevance of adult hippocampal neurogenesis. Front. Neurosci. 7, 63 (2013).
Yassa, M. A. & Stark, C. E. L. Pattern separation in the hippocampus. Trends Neurosci. 34, 515–525 (2011).
Kesner, R. P. in Prog. Brain Res. Vol. 163 (ed Helen E. Scharfman) 567–576 (Elsevier, 2007).
Suwabe, K. et al. Aerobic fitness associates with mnemonic discrimination as a mediator of physical activity effects: evidence for memory flexibility in young adults. Sci. Rep. 7, 5140 (2017).
Stark, S. M., Kirwan, C. B. & Stark, C. E. L. Mnemonic similarity task: a tool for assessing hippocampal integrity. Trends Cogn. Sci. 23, 938–951 (2019).
Hendrikse, J. et al. Regular aerobic exercise is positively associated with hippocampal structure and function in young and middle-aged adults. Hippocampus 32, 137–152 (2022).
Bullock, A. M., Mizzi, A. L., Kovacevic, A. & Heisz, J. J. The association of aging and aerobic fitness with memory. Front. Aging Neurosci. 10, 63 (2018).
Nauer, R. K., Schon, K. & Stern, C. E. Cardiorespiratory fitness and mnemonic discrimination across the adult lifespan. Learn. Mem. 27, 91–103 (2020).
Schwarb, H. et al. Aerobic fitness, hippocampal viscoelasticity, and relational memory performance. Neuroimage 153, 179–188 (2017).
Daugherty, A. M., Schwarb, H. D., McGarry, M. D. J., Johnson, C. L. & Cohen, N. J. Magnetic resonance elastography of human hippocampal subfields: CA3-dentate gyrus viscoelasticity predicts relational memory accuracy. J. Cogn. Neurosci. 32, 1704–1713 (2020).
Ekstrom, A. D. & Hill, P. F. Spatial navigation and memory: a review of the similarities and differences relevant to brain models and age. Neuron 111, 1037–1049 (2023).
Morris, R. G. M., Garrud, P., Rawlins, J. N. P. & O’Keefe, J. Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683 (1982).
Daugherty, A. M. et al. Path complexity in virtual water maze navigation: differential associations with age, sex, and regional brain volume. Cereb. Cortex 25, 3122–3131 (2015).
Moffat, S. D., Kennedy, K. M., Rodrigue, K. M. & Raz, N. Extrahippocampal contributions to age differences in human spatial navigation. Cereb. Cortex 17, 1274–1282 (2007).
Daugherty, A. M., Bender, A. R., Yuan, P. & Raz, N. Changes in search path complexity and length during learning of a virtual water maze: age differences and differential associations with hippocampal subfield volumes. Cereb. Cortex 26, 2391–2401 (2016).
Bartsch, T. et al. Focal lesions of human hippocampal CA1 neurons in transient global amnesia impair place memory. Science 328, 1412–1415 (2010).
Herting, M. M. & Nagel, B. J. Aerobic fitness relates to learning on a virtual Morris Water Task and hippocampal volume in adolescents. Behav. Brain Res. 233, 517–525 (2012).
van Praag, H., Kempermann, G. & Gage, F. H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 2, 266–270 (1999).
Kunz, L. et al. A neural code for egocentric spatial maps in the human medial temporal lobe. Neuron 109, 2781–2796.e2710 (2021).
Kunz, L. et al. Reduced grid-cell-like representations in adults at genetic risk for Alzheimer’s disease. Science 350, 430–433 (2015).
Maurer, A. et al. Effects of a 6-month aerobic exercise intervention on mood and amygdala functional plasticity in young untrained subjects. Int. J. Environ. Res. Public Health 19, 6078 (2022).
Upadhyay, N. et al. Regional cortical perfusion increases induced by a 6-month endurance training in young sedentary adults. Front. Aging Neurosci. 14, 951022 (2022).
American College for Sports Medicine. ACSM’s Health-Related Physical Fitness Assessment Manual (5th edition). (Lipincott Williams & Wilkins, 2017).
Arboleda-Serna, V. H., Feito, Y., Patino-Villada, F. A., Vargas-Romero, A. V. & Arango-Velez, E. F. Effects of high-intensity interval training compared to moderate-intensity continuous training on maximal oxygen consumption and blood pressure in healthy men: a randomized controlled trial. Biomedica 39, 524–536 (2019).
Zhu, L. et al. Brain structure, cardiorespiratory fitness, and executive control changes after a 9-week exercise intervention in young adults: a randomized controlled trial. Life (Basel) 11, 292 (2021).
Predovan, D. et al. Effect of aerobic exercise on white matter tract microstructure in young and middle-aged healthy adults. Front. Hum. Neurosci. 15, 681634 (2021).
Boullosa, D. et al. Effects of short sprint interval training on aerobic and anaerobic indices: a systematic review and meta-analysis. Scand. J. Med. Sci. Sports 32, 810–820 (2022).
Soshi, T. et al. Prefrontal plasticity after a 3-month exercise intervention in older adults relates to enhanced cognitive Performance. Cereb. Cortex 31, 4501–4517 (2021).
Amaral, D. G., Scharfman, H. E. & Lavenex, P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog. Brain Res. 163, 3–22 (2007).
Wisse, L. E. et al. Hippocampal subfield volumes at 7T in early Alzheimer’s disease and normal aging. Neurobiol. Aging 35, 2039–2045 (2014).
Puhlmann, L. M. C. et al. Association between hippocampal structure and serum Brain-Derived Neurotrophic Factor (BDNF) in healthy adults: a registered report. Neuroimage 236, 118011 (2021).
Eadie, B. D., Redila, V. A. & Christie, B. R. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J. Comp. Neurol. 486, 39–47 (2005).
Lafenetre, P., Leske, O., Wahle, P. & Heumann, R. The beneficial effects of physical activity on impaired adult neurogenesis and cognitive performance. Front. Neurosci. 5, 51 (2011).
Sorrells, S. F. et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555, 377–381 (2018).
Nogueira, A. B., Nogueira, A. B., Veiga, J. C. E. & Teixeira, M. J. Letter: human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Neurosurgery 83, E133–E137 (2018).
Nogueira, A. B. et al. Existence of a potential neurogenic system in the adult human brain. J. Transl. Med. 12, 75 (2014).
Kern, K. L., Storer, T. W. & Schon, K. Cardiorespiratory fitness, hippocampal subfield volumes, and mnemonic discrimination task performance in aging. Hum. Brain Mapp. 42, 871–892 (2021).
Wierenga, L. et al. Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. Neuroimage 96, 67–72 (2014).
Tamnes, C. K., Bos, M. G. N., van de Kamp, F. C., Peters, S. & Crone, E. A. Longitudinal development of hippocampal subregions from childhood to adulthood. Dev. Cogn. Neurosci. 30, 212–222 (2018).
Mu, S. H., Yuan, B. K. & Tan, L. H. Effect of gender on development of hippocampal subregions from childhood to adulthood. Front. Hum. Neurosci. 14, 611057 (2020).
Doeller, C. F., King, J. A. & Burgess, N. Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proc. Natl Acad. Sci. USA 105, 5915–5920 (2008).
Kunz, L. et al. Hippocampal theta phases organize the reactivation of large-scale electrophysiological representations during goal-directed navigation. Sci. Adv. 5, eaav8192 (2019).
Ben-Zeev, T. et al. Mild physical activity does not improve spatial learning in a virtual environment. Front. Behav. Neurosci. 14, 584052 (2020).
O’Mara, S. The subiculum: what it does, what it might do, and what neuroanatomy has yet to tell us. J. Anat. 207, 271–282 (2005).
Morris, R. G., Schenk, F., Tweedie, F. & Jarrard, L. E. Ibotenate lesions of hippocampus and/or subiculum: dissociating components of allocentric spatial learning. Eur. J. Neurosci. 2, 1016–1028 (1990).
O’Mara, S. M., Sanchez-Vives, M. V., Brotons-Mas, J. R. & O’Hare, E. Roles for the subiculum in spatial information processing, memory, motivation and the temporal control of behaviour. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 782–790 (2009).
O’Mara, S. M., Commins, S. & Anderson, M. Synaptic plasticity in the hippocampal area CA1-subiculum projection: implications for theories of memory. Hippocampus 10, 447–456 (2000).
Sharp, P. E. & Green, C. Spatial correlates of firing patterns of single cells in the subiculum of the freely moving rat. J. Neurosci. 14, 2339–2356 (1994).
Iglesias, J. E. et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage 115, 117–137 (2015).
Brown, E. M. et al. Test-retest reliability of FreeSurfer automated hippocampal subfield segmentation within and across scanners. Neuroimage 210, 116563 (2020).
Maurer, A. et al. Body image relates to exercise-induced antinociception and mood changes in young adults: a randomized longitudinal exercise intervention. Int. J. Environ. Res. Public Health 17, 6801 (2020).
Hautzinger, M., Bailer, M., Worall, H. & Keller, F. Beck Depressionsinventar [Beck Depression Inventory, German version]. (Huber, 1994).
Laux, L., Glanzmann, P. G., Schaffner, P. & Spielberger, C. D. Das State-Trait-Angstinventar (STAI) [State-Trait Anxiety Inventory, German version]. (Beltz, 1981).
Sheehan, D. V. et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59, 22–33 (1998).
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C. & Fagerstrom, K. O. The fagerstrom test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. Br. J. Addict. 86, 1119–1127 (1991).
Vorhees, C. V. & Williams, M. T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 1, 848–858 (2006).
Doeller, C. F., Barry, C. & Burgess, N. Evidence for grid cells in a human memory network. Nature 463, 657–661 (2010).
Coutrot, A. et al. Virtual navigation tested on a mobile app is predictive of real-world wayfinding navigation performance. PLoS ONE 14, e0213272 (2019).
Brenner, D., Stirnberg, R., Pracht, E. D. & Stocker, T. Two-dimensional accelerated MP-RAGE imaging with flexible linear reordering. MAGMA 27, 455–462 (2014).
Reuter, M., Schmansky, N. J., Rosas, H. D. & Fischl, B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61, 1402–1418 (2012).
Reuter, M., Rosas, H. D. & Fischl, B. Highly accurate inverse consistent registration: a robust approach. Neuroimage 53, 1181–1196 (2010).
Zhang, Y., Brady, M. & Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 20, 45–57 (2001).
Buckner, R. L. et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage 23, 724–738 (2004).
Sämann, P. G. et al. FreeSurfer-based segmentation of hippocampal subfields: A review of methods and applications, with a novel quality control procedure for ENIGMA studies and other collaborative efforts. Hum. Brain Mapp. 43, 207–233 (2022).
Cohen, J. A power primer. Psychol. Bull. 112, 155–159 (1992).
Acknowledgements
Our gratitude goes to the following students for supporting the data acquisition of the RUNSTUD study: Michael Decker, Sebastian Deckert, Milena Philippi, Marc Roderburg, Jonas Roos, Gereon Tacke, Lea Thomaschewski. Lukas Kunz received funding from the return program of the Ministry of Culture and Science of the State of North Rhine-Westphalia.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization of main study: H.B., L.S., J.A.M. and A.M.; Conceptualization of this substudy: H.B. and A.M.; Methodology: L.H., L.K., R.S., N.A., N.U. and A.M.; Software: L.K., N.A.; Formal analysis: L.H., L.K., N.U. and A.M.; Investigation: H.B., M.M., M.G., M.D., L.S., J.A.M. and A.M.; Resources: H.B., T.S., A.R., U.A. and N.A.; Data curation: M.M., M.G., T.N., and A.M.; Writing-original draft: H.B., A.M. and M.D.; Review and editing: all authors; Visualization: A.M.; Supervision: H.B. and A.M.; Project administration: A.M. All authors have read and agreed to the published version of the paper and there is no competing interest.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Sarah Aghjayan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Sahba Besharati and Manuel Breuer.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boecker, H., Daamen, M., Kunz, L. et al. Hippocampal subfield plasticity is associated with improved spatial memory. Commun Biol 7, 271 (2024). https://doi.org/10.1038/s42003-024-05949-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-05949-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.