Abstract
Exposure of testes to high-temperature environment results in defective spermatogenesis. Zebrafish exposed to high temperature exhibited apoptosis not only in germ cells but also in Leydig cells, as expected from studies using mice or salmon. However, the role of testicular somatic cells in spermatogenesis defects remains unclear. We found that in Leydig cells the Trpv4 gene encoding the temperature sensitive ion channel was specifically upregulated in high temperature. High temperature also reduced hormone synthesis in Leydig cells and led to a prompt downregulation of sperm motility. In the Trpv4 null mutant, neither Leydig cell-specific apoptosis nor decreased sperm motility was observed under high temperature. These results indicate that Leydig cell specific-apoptosis is induced via Trpv4 by high temperature. Notably this Trpv4-dependent mechanism was specific to Leydig cells and did not operate in germ cells. Because sperm exposed to high temperature exhibited compromised genome stability, we propose that temperature sensing leading to apoptosis in Leydig cells evolved to actively suppress generation of offspring with unstable genome.
Similar content being viewed by others
Introduction
Reproduction in an environment suitable for genome stability is important for the preservation of species. This requirement is more imperative in poikilotherms animals, in which gametes are directly exposed to undesirable temperatures. As a result, they may have evolved a system that rapidly adapts to such changes. In fish, for example, temperature is generally considered to be an important factor in determining the exact timing of gamete maturation and spawning1.
Inhibition of reproduction by high temperature is observed in a wide range of fish species2. High-temperature environment inhibits female ovulation and male spermatogenesis of Atlantic salmon (Salmo salar), which leads to arrest3. The suppression of ovulation by the high-temperature environment is due to decreased expression of 20β-hsd, which in turn inhibits maturation-inducing steroid conversion. However, the mechanism of spermatogenesis arrest with regard to the sensing of temperature in the testis remains unclear.
Testes in mammals as well as in fish show clear sensitivities to high-temperature environments. Mammalian testes exposed to temperature >37 °C display abnormal spermatogenesis by way of germ cell apoptosis through various signaling pathways4. A proposed model posits that high temperature causes damage to meiotic prophase I and damaged pachytene spermatocytes are eliminated at the checkpoints that monitor meiotic progression5. Checkpoints in multicellular organisms induce apoptosis in DNA-damaged cells and contribute to genome stability across generations. This mechanism contributes to the lower mutation rate of germ cells than somatic cells, which is important for stronger genome integrity6. The mitotic checkpoint functions also in zebrafish: high temperature applied to knockouts of Mps1, which is required for cell cycle fidelity and genome stability leads to the failure of checkpoint and production of aneuploid sperm7.
Leydig cells are found in the interstitial tissue of the testis and regulate spermatogenesis and reproductive function through the secretion of steroid hormones such as testosterone8. In mammalian models, hyperthermic stimulation of the testis by an artificially induced cryptorchidism leads to a decrease in Leydig cell activity as well as in germ cells9. Reduced activity of Leydig cells leads to decreased steroid hormone secretion10,11. High temperature also induces apoptosis not only in germ cells but also in Leydig cells12. However, the molecular mechanism and the significance of apoptosis in Leydig cells induced by high-temperature stimulation remain unexplored. In particular, the causal relationship between reduced Leydig cell activity and abnormal spermatogenesis due to the high-temperature environment is not clear.
In this study, we provide evidence that Leydig cells in zebrafish undergo apoptosis prior to germ cells when placed in high temperatures, and Trpv4 is involved in this process. Furthermore, we show that the Leydig cell-specific apoptosis decreased the synthesis of steroid hormones, which in turn impaired the motility of sperm whose genome integrity is compromised.
Results
High temperature affects Leydig cells prior to germ cells
To determine the appropriate condition for high-temperature stimulation in adult zebrafish whose optimal temperature is 28–30 °C, we first examined fish survival during 2 weeks of incubation at 33–39 °C. When placed in water <34 °C, mortality was not observed. In contrast, temperature >35 °C caused the death of incubated fish. Based on this observation, 34 °C was chosen as the temperature of stimulus in subsequent analyses (Supplementary Fig. 1). No abnormalities in spermatogenesis or morphological changes in the testes were observed after 2 weeks of rearing at 29 °C in the identical setup (Supplementary Fig. 2).
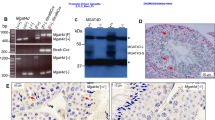
To analyze the effect of temperature stimulation on testicular structures and spermatogenesis, sections of the testes were observed by HE staining (Fig. 1a, Supplementary Fig. 3a). There are three developmental stages of spermatids: initial (E1), intermediate (E2), and final (E3)13. Temperature stimulation led to a decrease in spermatids, especially at E3 (circled in black lines), and the appearance of abnormal cells (circled in red lines). The abnormal cells had large nuclei relative to the cytoplasm and were different in appearance from any normal types of cells in germ cell development (Supplementary Fig. 4). Germ cells at pachytene, diplotene, and metaphase stages were present, but the number of spermatids was dramatically reduced in testis exposed to 34 °C. In normal zebrafish, germ cells at the identical differentiation stage are confined in a cyst14. In contrast, abnormal cells were observed within a single cyst containing meiotic germ cell at metaphase or spermatid after 1 and 3 days of exposure to 34 °C (Supplementary Fig. 4). After 7 days or 2 weeks at 34 °C, cysts with a mixture of normal and abnormal cells disappeared and only those with abnormal cells were observed (Supplementary Fig. 4). These results indicate that under high-temperature differentiation from metaphase to spermatid stages became abnormal.
a Top row: HE staining of testes. The black lines indicate areas containing E3 spermatid. The red lines indicate areas containing abnormal cell populations. Arrows indicate interstitial tissue. Interstitial areas decreased at 3, 7, and 14 days of temperature stimulation. Bottom row: Recovery of spermatogenesis and interstitial areas from temperature stimulation. The abnormal cell population disappeared, and E3 spermatid and interstitial areas were observed. b qRT-PCR analysis in testes. Values are the mean ± SEM (n = 4). *p < 0.01 compared with the 29 °C testes. c In situ hybridization of Insl3, a Leydig cell marker, in testes. d In situ hybridization of Hspa5. Black broken lines indicate interstitial cells and red broken lines indicate spermatocyte cells. e Localization of cleaved caspase 3. White broken lines indicate interstitial cells and red broken lines indicate spermatocyte cells. Scale bars are 20 μm.
In addition, there was a significant decrease in the interstitial tissue, which was observed as gaps between cysts. This tendency became more obvious as the duration of exposure to 34 °C became longer. Specifically, distinct Leydig cells were rarely detected after 2 weeks of exposure to 34 °C (Fig. 1a, upper panels).
Transfer of fish from 34 °C back to 29 °C resulted in the disappearance of the abnormal cell population and the appearance of interstitial cells in 7 days (Fig. 1a, lower panels). E3 spermatid appeared within 10 days of the transition to 29 °C, and spermatogenesis completely recovered to normal in 1 month. In zebrafish testes, undifferentiated type-A spermatogonia and differentiated type-B spermatogonia are present14. The type-A spermatogonia differentiates from E3 spermatid in 7 days13. There were no morphological abnormalities observed in type-A and type-B spermatogonia in testes treated at 34 °C temperature for 2 weeks (Supplementary Fig. 3b). Therefore, we expected that the transition to 29 °C would induce differentiation to the E3 spermatid within 1 week. However, differentiation into E3 spermatid was observed only after 10 days of recovery, which suggests the involvement of factors other than autonomous differentiation of germ cells.
We analyzed the expression of several groups of genes in testes exposed to 34 °C. Among them, genes in the Heat shock protein (Hsp) group showed significantly upregulated expression within 24 h of temperature stimulation (Fig. 1b). Hsps are genes that function as molecular chaperones and protect cells when exposed to stress conditions such as heat15. Most of the Leydig cell marker genes were significantly downregulated in the testes at 3 days of temperature stimulation. In contrast, the majority of marker genes for germ cells and Sertoli cells showed no change (Fig. 1b).
Based on the loss of E3 spermatid at 34 °C (Fig. 1a), we predicted a decrease in Odf3b, a spermatid marker16, which contrary to our expectation was not changed (Fig. 1b). To investigate why Odf3b expression was not decreased at 34 °C, we analyzed the localization of Odf3b expression in testes. Odf3b was expressed in spermatocytes and early spermatids but not in E3 spermatid at 29 °C (Supplementary Fig. 5). In contrast, it was expressed in spermatocytes and abnormal cells at 34 °C (Supplementary Fig. 5). These results suggested that temperature stimulation led to differentiation of Odf3b-expressing abnormal spermatid, which was consistent with histology (Fig. 1a).
Since temperature stimulation strongly affected Leydig cells, they were further analyzed by in situ hybridization using its marker Insl317. The region with positive signal decreased as the duration of 34 °C treatment became longer (Fig. 1c), which was consistent with HE staining and the expression analysis (Fig. 1a and b). Of note, the expression of Hspa5, involved in protein folding and assembly in the endoplasmic reticulum (ER)18 was induced by high temperature not only in Leydig cells but also in germ cells (Fig. 1d). These results suggest that the ER stress response to temperature stimulation occurred in both Leydig cells and germ cells.
Suspecting that apoptosis is involved in the different responses between Leydig cells and germ cells, we analyzed the localization of its marker, cleaved caspase 3. The signal was detected in the interstitial cells (circumscribed in white lines) after 1-day exposure to 34 °C and in spermatocytes (in red lines) after 3 days (Fig. 1e). These results indicated that apoptosis in response to 34 °C exposure is induced in Leydig cells prior to spermatocytes.
Temperature stimulation led to increased expression of Trpv4 in Leydig cells
To identify molecules involved in temperature sensing, we performed qRT-PCR for a group of Trp channel genes, sensor molecules for thermoreception19. Transient receptor potential (TRP) channels are expressed in sensory organs and play an important role in temperature sensitivity in zebrafish20,21. Expression of Trpv4, Trpm4b2, and Trpm4b3 was significantly increased by temperature stimulation (Fig. 2a). Among the three, the change was most evident for Trpv4, which was upregulated specifically in interstitial cells as shown by in situ hybridization (Fig. 2b). To further characterize these Trpv4-expressing cells, we performed double in situ hybridization using Leydig cell marker genes Insl3 and Trpv4. Remarkably, Trpv4 expression (blue) was limited to Insl3-positive cells (brown) in testes treated at 34 °C (Fig. 2c). These results show that temperature stimulation led to increased expression of Trpv4 specifically in Leydig cells.
a qRT-PCR analysis of Trp family genes in the testes. Values are the mean ± SEM (n = 3). **p < 0.01 and *p < 0.05 compared with the 29 °C testis. b In situ hybridization of Trpv4 in testes. c Double in situ hybridization of Trpv4 (blue) and Insl3 (yellow). The insets were magnified in the bottom rows. Scale bars are 20 μm (b and c: high magnification) and 50 μm (c: low magnification).
In Trpv4 KOs, apoptosis of Leydig cells was not induced by high-temperature
To clarify the function of TRPV4, a Trpv4 mutant was generated using the CRISPR-Cas9 system (Supplementary Fig. 6). An 8 bp deletion in exon 2 resulted in a frameshift causing a change in amino acid sequence and a premature stop codon. Trpv4−/− zebrafish were fertile and no abnormalities in spermatogenesis were observed under normal rearing conditions.
To analyze the effects of high-temperature treatment on testis structure and spermatogenesis in Trpv4−/−, testis sections were observed with HE staining (Fig. 3a, Supplementary Fig. 7). After 2 weeks at 34 °C, Trpv4−/− as well as Trpv4+/+ showed a decrease in E3 spermatid (black lines) and the appearance of abnormal cells (red lines). In contrast, distinct effects were observed in stromal tissue: Trpv4+/+ showed reduced interstitial tissue (arrows in Fig. 3a), while in Trpv4−/− it remained unaffected. Moreover, recovery of E3 spermatid was observed as early as 7 days after transferring the fish from 34 to 29 °C, earlier in Trpv4−/− than in Trpv4+/+.
a HE staining of testes. The upper panels show Trpv4+/+ and the lower panels show Trpv4−/−. Black line indicates areas of E3 spermatid. Red line indicates an abnormal cell population. Arrows indicate interstitial tissue. Interstitial tissue can be detected clearly in Trpv4−/− after 7 and 14 days of temperature stimulation. b qRT-PCR analysis of Leydig cell marker genes in testes. Values are the mean ± SEM (n = 4). *p < 0.01 compared with the 29 °C testis. c In situ hybridization in testes of Isl3, which is a Leydig cell marker. d In situ hybridization of Hspa5. Black broken lines indicate interstitial cells and red broken lines indicate spermatocyte cells. e Localization of cleaved caspase 3. White broken lines indicate interstitial cells and red broken lines indicate spermatocyte cells. Scale bars are 20 μm.
To examine further the effects of Trpv4 KO on Leydig cells, the expression of the Leydig cell marker genes was examined. Star, Insl3, and Cyp11c1 were significantly decreased by treatment at 34 °C in Trpv4+/+, while they remained unchanged in Trpv4−/− (Fig. 3b). Expression of Cyp17a1 and Hsd3β was significantly decreased in Trpv4−/− as well as Trpv4+/+ at 34 °C. These results suggested that steroid hormone biosynthesis in Trpv4−/− was affected to a lesser extent by temperature stimulation. Heterogeneity of Leydig cells in zebrafish, as was reported in adult mice22, may have affected the expression of marker genes in response to high-temperature stimulation. Noticeable difference was not observed for Hsp, germ cell markers or Sertoli cell markers (Supplementary Fig. 8). Correspondingly, Insl3(+) area decreased as the duration of treatment at 34 °C increased in Trpv4 +/+, while it remained unchanged in Trpv4−/− (Fig. 3c). Notably, the expression of Hspa5 showed no difference (Fig. 3d). Strong signals of cleaved caspase3 were detected in the interstitial cells (white lines) and spermatocyte (red lines) after 3 days of 34 °C treatment in Trpv4+/+. In Trpv4−/−, in contrast, cleaved caspase3 was restricted to the spermatocytes, and no clear signal in the interstitial cells was observed (Fig. 3e). These results indicate that temperature stimulation failed to induce Leydig cell apoptosis in Trpv4−/−.
High temperature impaired sperm motility via Trpv4 in Leydig cell
We analyzed the involvement of Trpv4 in the motility of mature spermatozoa by exposing adult males to 34 °C for 1 day. When the motility of sperm was analyzed under SI8000, which allows quantification of cell movement, a significant decrease in swimming velocity was observed in Trpv4+/+ but not in Trpv4−/− (Fig. 4a, c and Supplementary Movies 1–4, short movies showing representative cases for each genotype). The analysis of trajectories revealed that sperm from Trpv4+/+ after 34 °C exposure followed small circular trajectories, traveling shorter distance, which was not observed in Trpv4−/− (Fig. 4b). Steroid hormone (17α,20β,21-trihydroxy-4-pregnen-3-one (20β-S)) secreted from fish testis work on receptors expressed in sperms and control their motility23,24,25,26. 20β-S at 100 nM resulted in increased motility and improved trajectory of spermatozoa from Trpv4+/+ after 34 °C exposure (Fig. 4d–f and Supplementary Fig. 9).
a Heat map images of sperm motility. Red indicates high motility. Light blue indicates low motility. b Tracking of sperm swimming. The green line shows the trajectory of sperm in 3 s. c Pooled data of sperm motility in two groups. Values are the mean ± SEM (n = 8). *p < 0.01 compared with the 29 °C. d Heat map images of sperm motility with the addition of 100 nM 20β-S. Red indicates high motility; light blue indicates low motility. e Tracking of sperm swimming with the addition of 100 nM 20β-S. f Averaged sperm motility in added 20β-S. Values are the mean ± SEM (29 °C n = 7 and 34 °C n = 6). **p < 0.01 and *p < 0.05 compared with the 29 °C. g qRT-PCR analysis of the 20β-Hsd gene in the testes. Values are the mean ± SEM (n = 4). *p < 0.01 compared with the 29 °C testis. h In situ hybridization of 20β-hsd. Scale bars are 50 μm (a, b, d, and e) and 20 μm (h).
Based on these results, we hypothesized that the decrease of 20β-S secreted from Leydig cells after 34 °C treatment impaired sperm motility. The secretion of 20β-S in the testis was analyzed by LC–MS/MS. Unfortunately, 20β-S in the testes was below the detection limit (data not shown). We therefore used qRT-PCR to examine the expression of the 20β-hsd by qRT-PCR, an enzyme involved in the synthesis of 20β-S27. In Trpv4+/+, the expression was significantly decreased in testes at 3 days of temperature stimulation, while no difference was observed in Trpv4−/− (Fig. 4g). The result was further confirmed by in situ hybridization. In Trpv4+/+, 20β-hsd was expressed in the interstitial cells at 29 °C, but its expression disappeared as the incubation at 34 °C lasted longer. In contrast, Trpv4−/− showed clear expression in the interstitial cells even after temperature stimulation (Fig. 4h). Based on these results, we propose that the reduction of 20β-S synthesis, caused by decreased expression of 20β-hsd in Leydig cells, is responsible for the decrease in sperm motility induced by high temperature.
Indeed, when sperm isolated from the testis was directly exposed to high temperature, its motility did not decrease even at 40 °C (Supplementary Fig. 10). Although the duration of exposure to high temperature was shorter than the incubation of the whole fish (Fig. 4) due to technical limitations of maintaining isolated sperm in vitro, these results were consistent with the hypothesis that the endocrine regulation of Leydig cells, not autonomous regulation of sperm, determined the sperm motility in response to high temperature.
Sperm matured in a high-temperature environment leads to abnormalities in offspring
We hypothesized that zebrafish actively inhibit sperm motility in high temperatures so that embryos containing damaged cells, in particular damaged gametes, will not be generated. To test these predictions, we used flow cytometry to analyzed the chromosome content of sperm after 1 day of exposure to 34 °C. Regions of small cell size and low PI content were defined as mature sperm fractions (Supplementary Fig. 11). Sperm after 1-day exposure to 34 °C displayed a wider range of DNA content than sperm at 29 °C (Fig. 5a and b).
a Flow cytometric analysis of sperm DNA content. PI luminance values (30–42) were set as the P4 gate. b Percentage of sperm within the P4 gate. Values are the mean ± SEM (n = 6). *p < 0.01 compared with the 29 °C sperm. c Fertilization rates of 34 °C exposed male. Values are the mean ± SEM (29 °C n = 4 and 34 °C n = 5). **p < 0.01 and *p < 0.05 compared two groups. d Survival rates of embryos derived from 34 °C exposed males. Values are the mean ± SEM (29 °C n = 4 and 34 °C n = 5). *p < 0.01 compared with the 29 °C sperm. e Percentage of morphologically abnormal embryos derived from 34 °C exposed males. Values are the mean ± SEM (29 °C n = 4 and 34 °C n = 5). *p < 0.01 compared with the 29 °C sperm. f Images of embryos at 2 dpf and 6 dpf. Scale bars are 500 μm.
Males incubated at 34 °C for 1 day were mated with females normally reared and incubated at 29 °C. Fertilization rates were significantly lower for both WT and Trpv4−/− incubated at 34 °C than at 29 °C. However, in the 34 °C group, WT had significantly lower fertilization rates than Trpv4−/− (Fig. 5c). We propose that the Trpv4−/−, whose sperm velocity did not decrease even at 34 °C, had a decrease in fertilization rate smaller than that of WT (Supplementary Fig. 12). Among the fertilized eggs, there was no difference in survival up to 6 days post fertilization (dpf) (Fig. 5d). However, higher developmental abnormalities were observed in offspring from Trpv4+/+ and Trpv4−/− males exposed to 34 °C (Fig. 5e, f). Because a high frequency of developmental abnormalities occurs in offspring derived from aneuploid sperm in zebrafish7,28, these results suggested that some of the spermatozoa that matured in high-temperature environments have compromised genome stability. They also suggested that Trpv4 is not involved in the maintenance of sperm quality.
If fertilization occurs at high temperatures, generated embryos are also likely to develop in high temperatures. We therefore examined the effect of 34 °C water temperature on developing embryos of zebrafish. Embryos incubated at 34 °C resulted in high mortality rates and high developmental anomalies exceeding 70% at 1 dpf (Supplementary Fig. 13). It was clear that 34 °C is not a favorable temperature for normal embryonic development. However, it is of note that some embryos do develop normally and potentially grow up into fertile adults. These results indicated that zebrafish actively reduce sperm motility in order to suppress fertilization in high temperatures and thereby prevent the creation of embryos with compromised genome stability that can potentially be transmitted to offspring.
Temperature-dependent regulation of Leydig cells through Trp is species-specific
Finally, to examine whether this mechanism of Leydig cell sensitivity to high temperature is universally conserved among fish, we analyzed medaka (Oryzias latipes), which had an optimal temperature range distinct from that of zebrafish 29,30.
Similar to zebrafish, high-temperature stimulation in medaka testes leads to abnormal spermatogenesis31. However, the optimal temperature for spermatogenesis was also different between the two species. Medaka required exposure to higher temperatures compared to zebrafish to show reduced sperm motility (Supplementary Fig. 14a and b).
We performed qRT-PCR analysis of a group of Trp channels in the testes of medaka exposed to 39 °C. Hsp group was significantly upregulated in the temperature stimulation, but none of the genes in the Trp group was significantly upregulated (Supplementary Fig. 14c). Cleaved caspase3 was widely observed in the Leydig cells and germ cells especially spermatocytes after 1 day of exposure to 39 °C (Supplementary Fig. 14d). These results indicate that the induction of acute apoptosis in Leydig cells via Trpv4 by temperature stimulation is a phenomenon characteristic to zebrafish, and medaka may employ different molecules to reduce sperm motility in high temperature31.
Discussion
Multiple reports in mammals showed that germ cells are the main target of detrimental high temperatures in testes32. Our study using zebrafish revealed that high temperature induced apoptosis in Leydig cells prior to germ cells. Moreover, we showed that Leydig cells and germ cells in zebrafish have distinct mechanisms in sensing and responding to high temperatures. Trpv4 was involved in the Leydig cell-specific high-temperature sensitivity, which caused apoptosis, leading in turn to impaired sperm motility and reduced fertilization.
TRP channels were identified as sensor molecules for thermal reception, through which cellular responses to temperature changes are regulated33. Each TRP channel has a unique thermoreceptive range. Although TRP proteins are expressed also in the testes34, Trp in the zebrafish testis is poorly characterized. Trpv4 is strongly expressed in stressful environments35,36 and activated above 27–35 °C37, which fits the high-temperature condition used in this study. Trpv4 activation by endogenous and exogenous stimuli increases Ca2+ influx, resulting in an excess of intracellular free Ca2+38, and causes apoptosis in pathological conditions35,36,39. Trpv4 in zebrafish was studied by Amato et al. who showed the expression of this protein in sensory organs21. The lack of temperature sensing mechanism may also contribute to the phenotypes of Trpv4−/− we observed (Fig. 3). Taken together, these characteristics of Trpv4 fit our hypothesis that high temperature led to upregulation of Trpv4 in Leydig cells and caused apoptosis.
In zebrafish, as in mice, high-temperature stimulation caused abnormal spermatogenesis (Fig. 1a). Specifically, high-temperature treatment induced apoptosis in spermatocytes and abnormal differentiation from spermatocyte to spermatid, which was consistent with mice. Meiotic checkpoints cause spermatogenesis defects in mice following high-temperature stimulus and eliminate damaged spermatocytes40. A similar, sperm cell-autonomous mechanism seems to operate also in zebrafish. Hormone secretion from Leydig cells is unlikely to be essential for these cell-autonomous apoptoses because spermatogenesis failure was not distinguishable between Trpv4+/+ and Trpv4−/− (Fig. 3, Supplementary Fig. 12). Indeed, knockouts of steroid synthase genes in zebrafish can produce mature spermatozoa41,42,43,44, which was consistent with our hypothesis. On the other hand, the effect of hormones secreted from Leydig cells on certain aspects of spermatogenesis was suggested from our data. First, the transition from 34 to 29 °C led to earlier E3 spermatid differentiation in Trpv4−/− compared to Trpv4+/+. Second, expression of several genes including Insl3 did not change in Trpv4−/− exposed to 34 °C. In zebrafish, Insl3 was reported to act as a germline survival factor42. In Trpv4−/−, hormone secretion from Leydig cells was likely involved in promoting the rapid differentiation of E3 spermatid or survival of the remaining germ cells.
We propose that the Leydig cell-specific temperature-sensing mechanism comprises a system that suppresses fertilization in zebrafish under high-temperature conditions. In their natural habitat, zebrafish live in shallow tropical water with slow currents that are subject to frequent temperature fluctuations45, where water temperatures can rise to 38.6 °C46. High-temperature environments lead to severe abnormalities in early zebrafish development (Supplementary Fig. 13). Importantly, the maturation of spermatozoa in a high-temperature environment resulted in aneuploid spermatozoa (Fig. 5a). In addition, a high-frequency of developmental abnormalities were observed in offspring, even when incubated at 29 °C after fertilization (Fig. 5e and f). If gametes with compromised genomic integrity are transmitted to the next generation, the result will be detrimental to the species. Therefore, a mechanism to avoid fertilization in high-temperature environments will be advantageous.
Because the meiosis checkpoint controls the differentiation from metaphase to early spermatid, spermatozoa that have matured prior to temperature stimulation cannot be eliminated by the meiosis checkpoint. (Supplementary Figs. 4 and 5). Indeed, E3 spermatids were abundantly observed in the testis after 1-day incubation at 34 °C (Fig. 1a and Supplementary Fig. 3). Moreover, mature spermatozoa did not lose sperm motility in high temperatures (Supplementary Fig. 10). Therefore, mature sperm differentiated prior to temperature stimulation are expected to be motile with fertilization capabilities even at 34 °C. However, sperm motility and fertilization rate were significantly reduced by a 1-day exposure to 34 °C (Figs. 4a, c and 5c). Taken together, we propose that Trpv4-mediated reduction of 20β-S synthesis is the mechanism responsible for inhibiting fertilization of mature sperm which has already passed the meiosis checkpoint prior to exposure to high temperature.
Neither upregulated expression of Trp family genes nor Leydig cell-specific apoptosis at high temperatures was observed in medaka. Temperature stimulation at 39 °C resulted in reduced sperm motility and induction of apoptosis, especially in spermatocytes (Supplementary Fig. 14), while molecular changes identified in zebrafish were not observed in medaka. These results suggest that factors other than Trp and meiotic checkpoints are involved in the apoptotic response to high temperature in medaka. We propose that response to high temperature in Leydig cells was established as a system to actively suppress fertilization in zebrafish, whose adaptive water temperature range matched the molecular characteristics of Trpv4.
Methods
Fish
All experiments using animals in this study followed the guidelines of Osaka Medical and Pharmaceutical University. Adult zebrafish and medaka were maintained at 28.5 °C on a 10 h light:14 h darkness photoperiod. Zebrafish were bred from a RIKEN WT background and medaka strain was OKcab. Male adults 6–12 months old were used for experiments.
Temperature stimulation
Up to four individuals were temperature-stimulated in W22 × D12 × H11 cm tanks with 1.8 l of water, and water changes were performed daily. The tanks were placed in a water bath at 35 °C and oxygen was supplied by aquarium air stones. Temperature stimulation was interrupted for feeding from 12:00 to 13:00 daily. Exposure of isolated sperm was for 20 min (Supplementary Fig. 9).
Histology
Testes were fixed in Bouin solution, and 4 μm plastic sections were prepared using Technobit 8100 (Heraeus Kulzer). Samples were stained with hematoxylin solution for 10 min (Muto pure chemicals, Tokyo, Japan), and stained with 1% eosin (Muto pure chemicals, Tokyo, Japan) for 30 s. The mounted samples were imaged using an Olympus DP74 camera (Evident, Tokyo, Japan).
qRT-PCR
RNA samples were extracted from testes. According to the manufacturer’s instructions, 0.5 μg RNA template was used for reverse transcription to synthesize cDNA using a first-strand cDNA synthesis kit (ReverTra Ace, TOYOBO, Tokyo, Japan). The qPCR primers are listed in Supplementary Table 1. For amplification, the KOD SYBR qPCR/RT (TOYOBO, Tokyo, Japan) and ABI real-time system were used. Statistical significance was evaluated by two-tailed Student’s t-test.
In situ hybridization
RNA in situ hybridization (WISH) was carried out following a standard protocol47. The primers used to amplify the regions used for the probes are shown in Supplementary Table 1. Briefly, testes were fixed in 4% paraformaldehyde in 1× PBS overnight. On the following day, samples were hybridized at 65 °C overnight. Samples were further incubated with anti-digoxigenin-AP antibody solution (1:2000) overnight at 4 °C and stained with NBT/BCIP. The stained samples were imaged using an Olympus DP74 camera (Evident). Double in situ hybridization was carried out following a standard protocol48. The first probe was labeled with DIG and the second probe was labeled with FITC. After the first staining, incubation was performed with 0.1 M glycine–HCl pH 2.2 for 40 min to remove alkaline phosphatase activity.
Immunohistochemistry
Immunofluorescence staining was performed using an anti-Cleaved caspase 3 antibody (#9661, Cell Signaling Technology, USA). Alexa 488 was used as the secondary antibody. Nuclear DNA was stained with 4’,6-diamidino-2-phenylindol (DAPI). Stained samples were imaged using Leica SP8 (Leica, Germany).
Targeted genetic disruption of Trpv4 by CRISPR/Cas9
CRISPR/Cas9 target sites in Trpv4 were searched using CRISPR/Cas9 target online predictor (crispr.direct)49. Selected target sites are shown in Supplementary Fig. 5. Guide RNA was generated using a Guide-it sgRNA In Vitro Transcription kit (TAKARA, Tokyo, Japan). Cas9 mRNA (50 ng/μl) and sgRNA (25 ng/μl) were injected into one-cell stage embryos. Mutant allele was identified in F1 adult fish using the primer sets shown in Supplementary Table 1 and sequence analysis.
Sperm motility measurement
Zebrafish and medaka testes were diluted in fetal bovine serum (FBS). In zebrafish, sperm diluted in FBS were activated by adding an equal volume of breeding water immediately prior to measurement. Sperm motility was recorded as sequential 1024 × 1024-pixel phase-contrast images with a ×10 objective lens for 3 s at 300 frames per second (fps) using cell motion system SI8000 (Sony Corporation, Tokyo, Japan). Images were acquired with SI8000 view Software (Sony Corporation, Tokyo, Japan) and analyzed with SI8000R Analyzer Software. Appropriate amounts of 20β-S were added to the sperm suspension and incubated for 10 min before sperm motility was measured. 20β-S was purchased from Steraloids Inc. Statistical significance was evaluated by two-tailed Student’s t-test.
Flow cytometry
Testes were isolated from the adult zebrafish and dissociated by 0.2% collagenase. The cell suspension was fixated in 2% PFA and was stained with propidium iodide (PI) staining solution (50 μg/ml PI and 20 μg/ml RNase) for at least 10 min at room temperature in the dark. The cells were subsequently filtered through a 40-μm nylon mesh, and the suspension was analyzed using a FACSAria Fusion flow cytometer (BD Biosciences). Statistical significance was evaluated by two-tailed Student’s t-test.
Measurement of fertilization rate and survival rates in early embryogenesis
Embryos that developed past the epiboly stage were measured as fertilized eggs. Daily survival rates were measured relative to the number of eggs reaching the epiboly stage. Statistical significance was evaluated by two-tailed Student’s t-test.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The video data are available from the authors upon request. The source data behind the figures can be found in Supplementary Data 1.
References
Pankhurst, N. W. & Munday, P. L. Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 62, 1015–1026 (2011).
Pankhurst, N. W. & King, H. R. Temperature and salmonid reproduction: implications for aquaculture. J. Fish. Biol. 76, 69–85 (2010).
Taranger, G. L. et al. Effects of photoperiod, temperature and GnRHa treatment on the reproductive physiology of Atlantic salmon (Salmo salar L.) broodstock. Fish Physiol. Biochem. 28, 403–406 (2003).
Cobellis, G. et al. Spermatogenesis and cryptorchidism. Front. Endocrinol. 5, https://doi.org/10.3389/fendo.2014.00063 (2014).
Hirano, K. et al. Temperature sensitivity of DNA double-strand break repair underpins heat-induced meiotic failure in mouse spermatogenesis. Commun. Biol. 5, 504 (2022).
Milholland, B. et al. Differences between germline and somatic mutation rates in humans and mice. Nat. Commun. 8, 15183 (2017).
Poss, K. D., Nechiporuk, A., Stringer, K. F., Lee, C. & Keating, M. T. Germ cell aneuploidy in zebrafish with mutations in the mitotic checkpoint gene mps1. Genes Dev. 18, 1527–1532 (2004).
Zhou, R. et al. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell. Mol. Life Sci. 76, 2681–2695 (2019).
Kanter, M. & Aktas, C. Effects of scrotal hyperthermia on Leydig cells in long-term: a histological, immunohistochemical and ultrastructural study in rats. J. Mol. Histol. 40, 123–130 (2009).
Kühn-Velten, W. N. Rapid down-regulation of testicular androgen biosynthesis at increased environmental temperature is due to cytochrome P450c17 (CYP17) thermolability in Leydig cells, but not in endoplasmic reticulum membranes. Exp. Clin. Endocrinol. Diabetes 104, 243–249 (1996).
Munabi, A. K. et al. The effects of temperature on the activity of testicular steroidogenic enzymes. Steroids 43, 325–331 (1984).
Costa, G. M. J. et al. Higher environmental temperatures promote acceleration of spermatogenesis in vivo in mice (Mus musculus). J. Therm. Biol. 77, 14–23 (2018).
Leal, M. C. et al. Histological and stereological evaluation of zebrafish (Danio rerio) spermatogenesis with an emphasis on spermatogonial generations 1. Biol. Reprod. 81, 177–187 (2009).
Schulz, R. W. et al. Spermatogenesis in fish. Gen. Comp. Endocrinol. 165, 390–411 (2010).
Shan, Q. et al. Physiological functions of heat shock proteins. Curr. Protein Pept. Sci. 21, 751–760 (2020).
Oakes, J. A. et al. Ferredoxin 1b deficiency leads to testis disorganization, impaired spermatogenesis, and feminization in zebrafish. Endocrinology 160, 2401–2416 (2019).
Assis, L. H. C. et al. INSL3 stimulates spermatogonial differentiation in testis of adult zebrafish (Danio rerio). Cell Tissue Res. 363, 579–588 (2016).
Zhou, T. et al. Triclocarban at environmentally relevant concentrations induces the endoplasmic reticulum stress in zebrafish. Environ. Toxicol. 34, 223–232 (2019).
Peier, A. M. et al. A heat-sensitive TRP channel expressed in keratinocytes. Science (1979) 296, 2046–2049 (2002).
de Alba, G., López-Olmeda, J. F. & Sánchez-Vázquez, F. J. Rearing temperature conditions (constant vs. thermocycle) affect daily rhythms of thermal tolerance and sensing in zebrafish. J. Therm. Biol. 97, 102880 (2021).
Amato, V. et al. TRPV4 in the sensory organs of adult zebrafish. Microsc. Res. Tech. 75, 89–96 (2012).
Yokoyama, C. et al. Three populations of adult Leydig cells in mouse testes revealed by a novel mouse HSD3B1-specific rat monoclonal antibody. Biochem. Biophys. Res. Commun. 511, 916–920 (2019).
Tan, W., Pang, Y., Tubbs, C. & Thomas, P. Induction of sperm hypermotility through membrane progestin receptor alpha (mPRα): a teleost model of rapid, multifaceted, nongenomic progestin signaling. Gen. Comp. Endocrinol. 279, 60–66 (2019).
Tan, W. & Thomas, P. Activation of the Pi3k/Akt pathway and modulation of phosphodiesterase activity via membrane progestin receptor-alpha (mPRalpha) regulate progestin-initiated sperm hypermotility in Atlantic croaker1. Biol. Reprod. 90, 105 (2014).
Tubbs, C., Tan, W., Shi, B. & Thomas, P. Identification of 17,20β,21-trihydroxy-4-pregnen-3-one (20β-S) receptor binding and membrane progestin receptor alpha on southern flounder sperm (Paralichthys lethostigma) and their likely role in 20β-S stimulation of sperm hypermotility. Gen. Comp. Endocrinol. 170, 629–639 (2011).
Hanna, R. N. et al. Characterization and expression of the nuclear progestin receptor in zebrafish gonads and brain1. Biol. Reprod. 82, 112–122 (2010).
Nagahama, Y., Yoshikuni, M., Yamashita, M., Sakai, N. & Tanaka, M. Molecular endocrinology of oocyte growth and maturation in fish. Fish. Physiol. Biochem 11, 3–14 (1993).
Endoh, M. et al. Hybrid between Danio rerio female and Danio nigrofasciatus male produces aneuploid sperm with limited fertilization capacity. PLoS ONE 15, e0233885 (2020).
Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B. & Schilling, T. F. Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 (1995).
Shima, A. & Mitani, H. Medaka as a research organism: past, present and future. Mech. Dev. 121, 599–604 (2004).
Moreno Acosta, O. D., Boan, A. F., Hattori, R. S. & Fernandino, J. I. Notch pathway is required for protection against heat stress in spermatogonial stem cells in medaka. Fish. Physiol. Biochem. 49, 487–500 (2023).
Sinha Hikim, A. P. et al. Deciphering the pathways of germ cell apoptosis in the testis. J. Steroid Biochem. Mol. Biol. 85, 175–182 (2003).
Kashio, M. Thermosensation involving thermo-TRPs. Mol. Cell. Endocrinol. 520, 111089 (2021).
Kumar, P. G. & Shoeb, M. The role of TRP ion channels in testicular function. Adv Exp Med Biol. 704, 881–908 https://doi.org/10.1007/978-94-007-0265-3_46 (2011).
Liu, M. et al. TRPV4 inhibition improved myelination and reduced glia reactivity and inflammation in a cuprizone-induced mouse model of demyelination. Front. Cell. Neurosci. 12, 392 (2018).
Dong, Q. et al. Blockage of transient receptor potential vanilloid 4 alleviates myocardial ischemia/reperfusion injury in mice. Sci. Rep. 7, 42678 (2017).
Kashio, M. & Tominaga, M. TRP channels in thermosensation. Curr. Opin. Neurobiol. 75, 102591 (2022).
Baratchi, S. et al. The TRPV4 agonist GSK1016790A regulates the membrane expression of TRPV4 channels. Front. Pharmacol. 9, 10 (2019).
Olivan-Viguera, A. et al. Pharmacological activation of TRPV4 produces immediate cell damage and induction of apoptosis in human melanoma cells and HaCaT keratinocytes. PLoS ONE 13, e0190307 (2018).
Raina, V. B., Schoot Uiterkamp, M. & Vader, G. Checkpoint control in meiotic prophase: Idiosyncratic demands require unique characteristics. 281–315 https://doi.org/10.1016/bs.ctdb.2022.04.007 (2023).
Shang, G. et al. Steroidogenic acute regulatory protein and luteinizing hormone are required for normal ovarian steroidogenesis and oocyte maturation in zebrafish†. Biol. Reprod. 101, 760–770 (2019).
Crespo, D. et al. Insulin-like 3 affects zebrafish spermatogenic cells directly and via Sertoli cells. Commun. Biol. 4, 204 (2021).
Li, N., Oakes, J. A., Storbeck, K.-H., Cunliffe, V. T. & Krone, N. P. The P450 side-chain cleavage enzyme Cyp11a2 facilitates steroidogenesis in zebrafish. J. Endocrinol. 244, 309–321 (2020).
Oakes, J. A., Barnard, L., Storbeck, K.-H., Cunliffe, V. T. & Krone, N. P. 11β-Hydroxylase loss disrupts steroidogenesis and reproductive function in zebrafish. J. Endocrinol. 247, 197–212 (2020).
Spence, R., Gerlach, G., Lawrence, C. & Smith, C. The behaviour and ecology of the zebrafish, Danio rerio. Biol. Rev. 83, 13–34 (2007).
Morgan, R. et al. Are model organisms representative for climate change research? Testing thermal tolerance in wild and laboratory zebrafish populations. Conserv. Physiol. 7, coz036 (2019).
Nakamura, S. et al. Identification and lineage tracing of two populations of somatic gonadal precursors in medaka embryos. Dev. Biol. 295, 678–688 (2006).
Hurtado, R. & Mikawa, T. Enhanced sensitivity and stability in two-color in situ hybridization by means of a novel chromagenic substrate combination. Dev. Dyn. 235, 2811–2816 (2006).
Naito, Y., Hino, K., Bono, H. & Ui-Tei, K. CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 31, 1120–1123 (2015).
Acknowledgements
We are grateful to Mrs. Natsuko Okuda at OMPU for her excellent care of zebrafish and medaka used in this study. We are grateful to medical students at OMPU, Mr. Akihiro Tani, Ms. Yuri Ozaki, Mr. Tomohiro Hirayama, Mr. Takeyoshi Murakami, Mr. Kei Yotsumoto and Mr. Kakeru Terada in particular, for their assistance in some of the experiments. We thank Dr. Toshiya Nishimura for critically reading the manuscript. This research was partially supported by Grant-in-Aid for Scientific Research (19K06460, Y.Y.).
Author information
Authors and Affiliations
Contributions
Y.Y. carried out the experiment and analyzed the data. D.H. carried out the LC–MS/MS analysis. Y.Y. designed the study. F.O. supervised the study. Y.Y. and F.O. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Juan (I) Fernandino and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Frank Avila and Tobias Goris. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamamoto, Y., Hishikawa, D. & Ono, F. Trpv4-mediated apoptosis of Leydig cells induced by high temperature regulates sperm development and motility in zebrafish. Commun Biol 7, 96 (2024). https://doi.org/10.1038/s42003-023-05740-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-05740-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.