Abstract
Covering approximately 40% of land surfaces, grasslands provide critical ecosystem services that rely on soil organisms. However, the global determinants of soil biodiversity and functioning remain underexplored. In this study, we investigate the drivers of soil microbial and detritivore activity in grasslands across a wide range of climatic conditions on five continents. We apply standardized treatments of nutrient addition and herbivore reduction, allowing us to disentangle the regional and local drivers of soil organism activity. We use structural equation modeling to assess the direct and indirect effects of local and regional drivers on soil biological activities. Microbial and detritivore activities are positively correlated across global grasslands. These correlations are shaped more by global climatic factors than by local treatments, with annual precipitation and soil water content explaining the majority of the variation. Nutrient addition tends to reduce microbial activity by enhancing plant growth, while herbivore reduction typically increases microbial and detritivore activity through increased soil moisture. Our findings emphasize soil moisture as a key driver of soil biological activity, highlighting the potential impacts of climate change, altered grazing pressure, and eutrophication on nutrient cycling and decomposition within grassland ecosystems.
Similar content being viewed by others
Introduction
Grassland systems covering approximately 40% of the world’s terrestrial surface, encompass a wide variety of habitats for soil organisms1,2, which play key roles in delivering ecosystem functions such as nutrient cycling and decomposition3,4,5,6. In this context, the key players are soil microorganisms and detritivores such as earthworms, isopods, millipedes, and enchytraeids, which primarily feed on litter and organic materials. Their collective efforts break down organic matter, thus supplying vital nutrients to plants7. Soil organism activity is strongly driven by temperature, soil moisture8,9,10,11, and global change factors, including increased nutrient inputs and alterations in the range, abundance, and distribution of aboveground herbivores. However, we lack a broad understanding of how nutrient inputs and herbivory influence soil communities and ecosystem functions in grasslands. At the same time, such soil organisms may be important mediators of ecosystem responses to global change2,12,13. Further, a lack of spatially replicated studies means that we cannot predict how plant productivity, grazing, or local abiotic characteristics may mediate nutrient and herbivory effects on soil organisms14.
Herbivores can play a crucial role in shaping grasslands by facilitating diverse plant communities and maintaining ecosystem functioning14. For example, wild herbivores may selectively consume abundant plant species, altering species composition15,16,17 and can contribute to maintaining plant diversity by reducing competition for light18. Moreover, herbivores impact nutrient cycling in grasslands by consuming live plant material and modifying the quantity and quality of organic inputs to the soil, e.g. via excreta, and via changes in soil abiotic conditions14,19,20. At the same time, large native herbivore densities may be reduced via hunting or land conversion, and in many cases, they are replaced by large numbers of domestic livestock21,22. Soil communities, processes, and structure are strongly affected by wild and domestic herbivores, with important consequences for soil biological activity and ecosystem multifunctionality14,23,24,25. Herbivores may enhance soil biological activity by depositing easily-degradable dung and urine or, particularly under fertile conditions, inducing compensatory growth19,26,27,28. In contrast, in relatively unproductive systems, grazers may preferentially feed on the few available nutrient-rich plants, on which many soil organisms also depend, resulting in poorer quality of litter, which reduces biological activity15,19,29,30. Additionally, aboveground herbivores may create harsh environmental conditions for soil organisms through soil compaction, negatively affecting pore space and water infiltration as well as increasing the cover of bare soil, resulting in high temperature fluctuations compared to vegetated areas31,32. At the same time, the interaction between herbivory and nutrients can be context-specific, as it may vary based on the specific plant species and local site conditions33.
Predictions suggest that the disruption of the nitrogen cycle could cause nitrogen (N) deposition to double in the future34,35. The same applies to phosphorus (P) inputs, which have globally increased compared to preindustrial levels36,37. The growth and biomass production of plants depend on nutrients such as nitrogen, phosphorous, and potassium, and most grasslands are limited in productivity by nutrient inputs38,39. Nitrogen inputs may increase the activity of soil organisms by increasing the amount and quality of plant material that enters the soil system10,20, but have also been shown to reduce detritivore activity40. The same applies to soil microbes, as long-term nitrogen inputs have been shown to have negative effects41. While the effects of phosphorous inputs on microbial activity remain less understood, it is known that phosphorous limitation can impede decomposition41,42,43. Globally, nitrogen to phosphorous ratios are increasing, leading to a prevalence of phosphorous limitation in soils36. This limitation can further inhibit microbial activity, which in turn can impact biological decomposition processes44. Additionally, although soil microbes are generally less susceptible to potassium deficiency than plants45, they still benefit from increased nutrient inputs, including potassium and micronutrients from plants that have sufficient nutrient supply. Given these context-dependent effects of nutrient addition and herbivory on soil processes, we need standardized manipulations of herbivores and nutrients across experimental and environmental gradients.
To improve our understanding of how fertilization and herbivory may alter ecosystem functioning belowground, we investigated the effects of nutrient enrichment (NPK fertilization) and herbivore reduction on soil microbial and detritivore activity across grasslands worldwide. This globally-coordinated study of soil biota was carried out within the Nutrient Network experiment46, with sites in North and South America, Europe, Asia, and Australia that represent a wide range of grassland habitats and environmental conditions (Fig. 1a; Table S1). In 2015, we used standardized bait (bait lamina strips) at 18 sites to assess soil detritivore feeding activity47, and analyzed soil samples from 26 sites for soil microbial activity (microbial respiration)48. Throughout the manuscript, we use the term ‘biological activity’ to encompass both activities. We used structural equation models to test which biotic (plant community properties49) and abiotic properties (soil water content11) determine soil biological activity worldwide. We hypothesized that (1) reducing aboveground herbivores would result in a decrease in belowground activity rates. Furthermore, we expected (2) the impact of added nutrients on soil biological activity would depend on carbon inputs, with increased plant biomass due to nutrient additions being associated with higher soil biological activity. With the two treatments in combination (3), the positive effect of nutrients on soil activity would be stronger than the negative effect of reduced herbivory, leading to a net increase in soil biological activity. Here, we expected that the positive effect of nutrients on soil activity would be stronger than the negative effect of reduced herbivory.
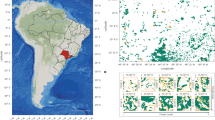
a Global map of all participating sites in the study. Red dot = data on soil microbial and detritivore activity (n = 18 sites); blue dot = data on soil microbial activity only (n = 26 sites). b, c Show two figures where we tested the effect of NPK fertilization, herbivore reduction, and the interactive effect of NPK fertilization and herbivore reduction on soil detritivore activity (log-scaled) and soil microbial activity (log-scaled). Points are raw observations; error bars indicate 95% confidence intervals. Significance levels: (*) p-value = 0.06, ns not significant.
Results
Effects of nutrient addition and herbivore exclusion
Soil detritivore feeding activity ranged from 0.94% to 77% of available bait substrate removed (Fig. S1a). Soil microbial respiration ranged from 0.27 µl O2 h-1 g-1 soil dry weight to 8.93 µl O2 h-1 g-1 soil dry weight. (Fig. S1b). Nutrient addition had no significant effect on detritivore feeding activity (F = 0.08; p = 0.78) and soil microbial activity (F = 0.94; p = 0.333). Despite high among-site variation (Fig. S2), herbivore reduction had a positive effect on detritivore feeding (Fig. 1b; F = 3.60; p = 0.06), resulting in higher activity levels when herbivores were reduced (+16.7%). At the same time, herbivore reduction did not affect soil microbial activity (Fig. 1c; F = 0.29; p = 0.59) Similarly, there was no interactive effect of NPK fertilization and herbivore reduction on detritivore (F = 0.21; p = 0.65) and microbial (F = 0.63; p = 0.43) activity.
Structural equation model analyses
The site-specific environmental conditions and treatments also had strong effects on the soil environment and the associated plant community, which became more apparent when the interdependence of variables was considered. Mean annual precipitation (MAP) and soil water content were positively correlated (Fig. S6) and structural equation modeling shows that MAP and soil water were positively associated with soil detritivore and microbial activity (Figs. 2a and 3a). At the same time, soil biological activity rates increased with higher amounts of MAP and soil water content, regardless of other treatment conditions (Figs. S4a, b and S5). Reflecting our results from linear mixed-effects models, nutrient addition had no direct effect on soil biotic activity. However, our SEM model revealed that herbivore reduction directly increased detritivore activity and indirectly increased activity of all soil microbes and detritivores via increasing soil water content (Table S3; Figs. 2a, c and 3a, c). Plant biomass, which increased with site MAP and both NPK and herbivore reduction treatments, was related with lower soil microbial activity (see also Fig. S6). At the same time, we found detritivore and microbial activities to be significantly positively correlated (Fig. 4; F = 9.15; p = 0.003).
a Soil detritivore activity as a best-fit Structural Equation Model showing the effects of NPK fertilization and herbivore reduction (Fisher’s C = 1.88; P = 0.758; d.f. = 4; 18 sites). Black arrows indicate significant positive and red arrows indicate significant negative effects in the model (P < 0.05). Dashed gray arrows indicate non-significant effects (P > 0.05) that remain in the model based on AIC. Dark gray double-headed arrows indicate paths that were treated as correlated errors in the model. Arrow widths are proportional to their effect sizes. Numbers along the arrows are standardized path coefficients. Marginal R2m: model variation explained by fixed effects; conditional R2c: model variation explained by both fixed and random effects. Significance levels: *p < 0.05; **p < 0.01; ***p < 0.001. b Direct, indirect, and net effect of MAP on soil detritivore activity, and c direct, indirect, and net effect herbivore reduction on soil detritivore activity.
a Soil microbial activity as a best-fit Structural Equation Model showing the effects of NPK fertilization, herbivore reduction (A/C = 77.9, Fisher’s C = 1.932; P = 0.381; d.f. = 2; 26 sites). Black arrows indicate significant positive and red arrows indicate significant negative effects in the model (P < 0.05). Dashed gray arrows indicate non-significant effects (P > 0.05) that remain in the model based on AIC. Dark gray double-headed arrows indicate paths that were treated as correlated errors in the model. Arrow widths are proportional to their effect sizes. Numbers along the arrows are standardized path coefficients. Marginal R2m: model variation explained by fixed effects; conditional R2c: model variation explained by both fixed and random effects. Significance levels: *p < 0.05; **p < 0.01; ***p < 0.001. b Direct, indirect, and net effect of MAP on soil microbial activity, and c direct, indirect, and net effect herbivore reduction on soil microbial activity, and d direct, indirect, and net effect of NPK fertilization on soil microbial activity (scale of b) differs from c and d.
Discussion
We conducted a globally-distributed experiment assessing the responses of soil biological activity to nutrient addition and herbivory at 26 sites, spanning five continents and multiple grasslands. Soil microbial and detritivore activity were associated with similar drivers at the global scale. Soil biological activity increased with MAP and soil moisture, suggesting that future climatic changes related to alterations in the amount and frequency of precipitation as well as evapotranspiration50 may have major consequences for grassland ecosystem functioning. To determine whether this pattern is causal or due to covariation with other variables such as geological and historical factors, it will be necessary to conduct future experimental manipulations. Both soil biological activity measures are tightly linked to decomposition processes that determine carbon sequestration and release11,51, and therefore play a key role in grassland carbon cycles52. In addition, herbivore presence was associated with lower soil moisture which may amplify effects of a drier climate. Moreover, MAP, NPK addition, and herbivore reduction had indirect negative effects on soil microbial activity by increasing plant biomass. This indicates that plant community-mediated changes in soil microbial communities and functions depend on abiotic and biotic conditions. This study improves our mechanistic understanding of factors determining soil biological activity globally, which is crucial to predict belowground ecosystem functioning in a changing world and to adopt measures to preserve grassland systems2,46,53,54.
In line with our hypothesis (1), we found a consistent overall positive effect of herbivore reduction on soil detritivore activity across all sites. In addition, through our SEM analysis, we observed that herbivore reduction enhanced soil detritivore activity directly and also indirectly via an increase in soil water content. However, while herbivore reduction had no direct effect on soil microbial activity, it also indirectly increased microbial activity via increases in soil water content. It has long been recognized that soil organisms strongly depend on soil moisture (e.g., residing on water films within the soil pore system)11,55,56,57,58. Confirming this, our results highlight the role of water availability for both measures of soil biological activity, with higher activity levels at sites with higher MAP and soil water content. Previous studies have found herbivores to reduce soil water content59 and to have negative effects on soil organisms, especially in unproductive ecosystems17,19,53,60,61. Our results suggest that a reduction in soil activity with herbivory is the dominant pattern in grasslands. This finding is consistent with other studies reporting decreased soil respiration in response to lower soil water content62,63,64. In our study, soil water content had a significantly larger effect on soil microbial activity compared to detritivore activity. This might be attributed to the fact that the soil water content data were better aligned with the measure of soil microbial activity. However, it is also possible that detritivores are less influenced by water content than microorganisms due to their ability to move to deeper soil layers65. This adaptability allows them to sustain their activity even in drier conditions. In support of this, Sagi et al.66 discovered that the primary litter decomposition in the Negev desert during summer was driven by a woodlice species, in contrast to microbes which lacked the necessary water for growth.
Other possible mechanisms for direct negative impacts of (especially larger) herbivores on soil detritivores and thus the positive effect when they were excluded, entail physical disturbances like trampling and soil compaction53,67,68. These result in higher bulk density and reduced connectivity of soil pores69 that normally ensure water infiltration and air permeability70,71. Such a reduction in soil pore space has been shown to reduce the abundance and diversity of soil arthropods and annelids69,72,73. For example, Collembola and enchytraeids strongly depend on macropores in their living environment, have hardly any ability to move through compacted soil, and may thus experience reduced access to food resources, consequently inhibiting their feeding activity69. However, even soil animals with considerable burrowing abilities, like earthworms, have been shown to be negatively affected by soil compaction74. Indeed, we found some evidence for a significant positive relationship between soil microbial and detritivore activity and soil porosity across a subset of sites (Fig. S8). However, given that only a subset of the sites could be considered for this analysis, this topic needs to be addressed in future research.
At the same time, we observed increases in plant biomass that were associated with herbivore reduction, nutrient addition, and higher levels of precipitation75. It is well-established that vegetation cover helps to maintain high levels of soil biological activity, as evidenced by previous studies76,77,78,79,80, which is consistent with our own findings. However, higher plant biomass also led to declines in soil microbial activity which is, in contrast to other studies reporting positive effects of higher plant biomass on soil biological activity via bottom up effects of increased rhizodeposition19,81,82,83. There are several possible explanations. On one hand, enhanced plant growth could potentially result in higher transpiration rates84,85, ultimately leading to a reduction in soil water content over time. On the other hand, herbivore reduction also led to a less diverse plant community, which could also decrease microbial respiration. It is also possible that soil microbial communities have to compete with plants for nutrients, possibly leading to reduced respiration rates. Follow-up studies are needed to relate environmental change-induced alterations in soil microbial communities to ecosystem functions, using standardized, replicated methods to increase the generality and robustness of such experiments86,87.
In contrast to our hypotheses (2 & 3), we did not observe an either significant effect of nutrient addition soil biological activity or a significant interaction between nutrient addition and herbivory. Although we applied NPK at high levels, we did not detect any direct fertilization effect, suggesting that availability of mineral nutrients is not the main determinant driving soil biological activity in grasslands. However, soil community responses and functions can be diverse and context-dependent88. Previous studies have shown that nutrient addition can alter the soil community by changing pH, porosity, organic fractions, and increasing decomposition, but responses of soil microbial respiration and biomass to NPK addition are highly variable25,40,41,89,90,91. However, our findings show a decline in soil microbial activity due to nutrient addition in global grasslands, alongside an increase in total plant biomass. Nutrient addition altered plant communities through by increasing total plant biomass and reducing plant species richness. Similar effects have been reported by multiple studies46,92,93,94,95,96,97. As nutrient addition has been shown to decrease soil organic matter stabilization, we speculate that our results may be connected to shifts in plant or soil microbial communities that influence the recalcitrance of organic matter and its microbial processing98.
Assessing soil microbial activity for a global scale study entailed some constraints that could have also influenced our findings. Measuring soil microbial activity involved homogenizing, sieving, and shipping soil samples to a central laboratory, which includes disruption of soil aggregates, along with potential changes in microbial activity due to shipping conditions. Additionally, assessing microbial activity under controlled temperature conditions, differing from the natural environment, might have influenced the correlations observed with mean annual temperature (MAT). Similarly, the bait lamina test, offering an on-site approach to assess detritivore activity, has faced recent critique regarding its use of standardized substrates as accurate indicators of local plant litter-driven decomposition99 even though this concern was not directly tested for bait lamina strips.
Overall, our results highlight that reductions in mean annual precipitation or prolonged drought periods may reduce soil biological activity that is key for the provisioning of essential ecosystem functions like nutrient cycling and decomposition52,100. These expected changes in climate could be further amplified by alterations in the abundance and identity of herbivores as well as nutrient inputs, with complex feedback mechanisms, including shifts in local plant community composition and productivity, as well as abiotic factors like soil compaction. Nutrient addition did not directly affect soil biological activity across global grasslands, emphasizing the importance of an indirect effect via plant biomass that should be considered in future studies. These novel insights into the global drivers of soil biological activity stress the complex interplay between different components of anthropogenic change that may alter above-belowground interactions and thus the functioning of global grasslands.
Methods
Experimental design and included sites
Fieldwork was conducted in 2015 within the Nutrient Network Global Experiment (www.nutnet.org)46 at 26 sites (see Fig. 1a and Table S1). The sites are located in North and South America, Europe, Asia, and Australia and are all dominated by herbaceous or low-statured vegetation (hereafter referred to as grasslands). Moreover, sites cover wide environmental gradients with elevations ranging from 6 m to 4.241 m a.s.l., mean annual temperatures from −3.3 °C to 22.4 °C, and mean annual precipitation from 324 mm to 1678 mm (Table S1). The experiments were set up at different times in 2009–2014 (for details see Table S1).
For our study, we sampled plots at each site, which were randomly assigned to one of four: Control, nutrient addition, fenced, nutrient addition and fenced. Each treatment was replicated three times at each site, leading to a total of 12 plots. Fences excluded aboveground herbivores weighing more than 50 g. The plots were 5 × 5 m in size and NPK plots received a fertilization treatment of nitrogen (N), phosphorus (P), and potassium (K). Nutrient addition rates and sources are: 10 g N m-2 * year-1 as timed-release urea [(NH2)2CO], 10 g P m-2 * year-1 as triple-super phosphate [Ca(H2PO4)2], 10 g K m-2 * year-1 as potassium sulphate [K2SO4]. Additionally, 100 g m² of a micronutrient mix of Fe (15%), S (14%), Mg (1.5%), Mn (2.5%), Cu (1%), Zn (1%), B (0.2%), and Mo (0.05%) were applied once at the start of the experiment. In contrast, control plots did not receive additional nutrients and represent ambient soil conditions. The fences for herbivore reduction were of 2.3 m height, with few sites having physical constraints that required fence modification. They were set up with a 1 cm woven wire mesh extending 0–90 cm aboveground and a 30 cm outward-facing flange stapled to the ground to exclude smaller digging animals. To reduce possible impacts of neighboring plots, all plots are separated by walkways of at least 1 m width. All sampling occurred in a single, randomly selected, 2.5 × 2.5 subplot of each plot. Further details on the experimental set up, standardized sampling protocols, and nutrient sources are described in Borer et al.46.
Plant data
Following the standardized Nutrient Network protocol, total aboveground plant biomass was clipped at peak biomass within two 0.1 × 1 m strips per plot, whose locations are changed each year (see Borer et al., for details). Sorted plant material was dried at 60 °C to a constant mass and weighed to the nearest 0.01 g. For our analyses, we used data on total plant biomass (i.e., the sum of dead and live plant biomass) from 2015 (i.e., the year of the study) as a proxy for plant-derived inputs to the soil, such as rhizodeposits and plant litter. Plant species richness was assessed on-site in a permanent 1 × 1 m quadrat located in the focal subplot in each plot.
Climate variables
Data on mean annual precipitation (MAP in mm) and mean annual temperature (MAT in °C) were derived from the WorldClim database (version 1.4; Hijmans et al.). Values were interpolated at high resolution from meteorological stations with 10 to 30 years of data101.
Soil sampling
Soil invertebrate feeding activity was assessed at 16 sites using the bait lamina test (Terra Protecta GmbH, Berlin, Germany), which is commonly used as a rapid ecosystem function assessment method11,47. The bait strips are made of PVC (1 mm × 6 mm × 120 mm) and have 16 holes (1.5 mm in diameter). Holes were filled with an artificial organic bait substrate, which was prepared according to the recommendations of Terra Protecta, consisting of 70% cellulose powder, 27% wheat bran, and 3% activated carbon. The bait substrate is primarily consumed by soil collembolas, enchytraeids, and earthworms76,102; microbial activity plays a minor role in bait loss103,104,105. The bait strip assessment was done by the principal investigator of each site. The bait strips were inserted vertically into the soil with the uppermost hole just beneath the soil surface. A steel knife was used to create a slot in the soil, before the strips were inserted. Five strips were spaced 15 cm apart within each plot to account for within-plot spatial heterogeneity. After three to six weeks of exposure, the bait strips were removed from the soil and directly evaluated in the field. Each hole was rated as 0 (no invertebrate feeding activity), 0.5 (bait material partly consumed), or 1 (bait material completely consumed), based on visual inspection. Thus, soil invertebrate feeding activity could range from 0 (no feeding activity) to 16 (maximum feeding activity) per strip. Mean bait consumption of the five strips was calculated per plot prior to statistical analyses and expressed as a percentage. Timing variations resulted from the substantial environmental differences, as in some cases, short exposure intervals did not yield discernible changes (for detailed exposure time please see Table S1).
Soil for microbial data was collected from 26 sites six weeks before peak plant biomass production (local site coordinators chose specific dates, as seasonality varied across different latitudes) by taking three subsamples per plot (using a soil corer with 5 cm diameter and 12 cm depth), which were then homogenized and sieved using a 2 mm mesh. All soil samples to Anita Risch in Switzerland following a standardized protocol. A subset of these samples was then shipped to a centralized lab at the German Centre for Integrative Biodiversity Research in Leipzig, Germany. We ensured sample quality during transit by using postal services with temperature control and fast shipping methods. Here, we took approximately 6 g of fresh soil to measure basal respiration (without the addition of substrate) at hourly intervals for 24 h at 20 °C using an O2-microcompensation system48. We used four different O2-microcompensation devices to measure all samples simultaneously. Basal respiration, as a measure of soil microbial activity, was then calculated as the mean O2 consumption rate 14–24 h after the start of the measurements (µl O2 h-1 per g soil dry weight), as the machine needs some time to measure stable values over an extended period77. In addition, soil water content [%] was calculated as the difference between the weight of the fresh soil sample and the weight of the soil sample per plot after they were dried for at least 48 h at 70 °C. Soil water content was significantly positively correlated with soil water holding capacity (R² = 0.61, p < 0.001). Soil porosity was determined as described in Risch et al.25, but was only available for a subset of 15 sites.
Statistics and reproducibility
To assess the effects of fertilization, reduced density of vertebrate herbivores, and their interaction on soil detritivore feeding and microbial respiration across all sites without accounting for abiotic factors and plant data, we employed linear mixed-effects models using the lmer function from the R-package “lme4106”. The model’s random intercepts were organized based on two factors: (1) block nested within site, and (2) the type of O2-microcompensation device (for soil microbial data) or field exposure duration (for detritivore data), as some sites had a longer exposure time due to logistical constraints. We also tested a model with treatment duration years as a fixed effect, but found that treatment duration had no significant impact on soil microbial and detritivore activity, and consequently excluded “treatment duration” from our explanatory parameters. To account for the non-normality of our response variables, we log-transformed data prior to our analysis. Figure 1b, c are based on mixed-effects model fits extracted using the package “ggeffects107”.
We used structural equation modeling (SEM) to disentangle direct and indirect pathway effects by which fertilization and herbivore reduction affected the activity of soil organisms. In determining the environmental variables for our SEM approach, we considered factors that could offer meaningful insights into the dynamics of soil microbial and detritivore activity. These variables were selected a priori based on their established influence on soil microbial and detritivore activity, as well as their potential to mediate treatment effects. Our choices were guided by existing literature in the field. Given that plant community composition and biomass are strong predictors of soil microbial and detritivore activity and are thus likely to mediate the treatment effects, we included plant species richness and total plant biomass in the SEM (Figs. 2 and 3). As soil organisms are highly dependent on soil moisture108, we included soil water content as a key abiotic driver. We also chose to include mean annual precipitation (MAP) as another exogenous variable, as it was correlated with soil moisture (Fig. S7) with the two soil activity variables (Figs. 2 and S4a, b) and should have long-lasting effects on soil conditions that are also relevant for our snapshot assessments. We further selected mean annual temperature as another exogenous variable. However, the relationship between mean annual temperature (MAT) and microbial activity was not statistically significant (p = 0.14), and a similar non-significant trend was observed with detritivore activity (p = 0.93) (see also Fig. S4c, d). Although MAT displayed a positive correlation with plant species richness, this association did not extend to the other variables in our SEM model (as illustrated in Figs. 2 and 3). Consequently, we decided to exclude MAT from the final model. The framework of the “piecewiseSEM” R package109 allowed us to test for interactive treatment effects and to account for the hierarchical study design by including random effects in the models. We also investigated the effects of soil pH and individual effects of living and dead plant biomass on soil microbial and detritivore activity within the model. However, these data were only available for a small subset of sites and as we found no significant direct or indirect effects on biological activity, we excluded them in the final model.
The single models that were incorporated in the SEM were built using LMMs (Table S2). The assumptions of the LMMs were checked by plotting frequency distributions of each variable and the variance structure of all models using residual plots for homogeneity and quantile-quantile plots for normality (i.e., no correlation between the residuals and the fitted parameters of the model). To meet model assumptions, plant biomass, plant species richness, soil water content, and detritivore activity were log-transformed. The relationship between plant richness and total plant biomass was included as a correlated error term due to reciprocal effects110.
The number of variables was reduced from the conceptual model using the Akaike Information Criterion (AIC) that is implemented in the “piecewiseSEM” package. Standardized coefficients are reported for each path of the final model (Tables S3 and S4, Figs. 2a and 3a). The overall fit of the models was evaluated by using Shipley’s test of d-separation obtained through Fisher’s C statistic. Correlations were performed between soil microbial activity and soil detritivore activity. To examine the impact of herbivore-induced changes in soil structure on biological activity, we analyzed the correlation between soil porosity (a measure of soil compaction influenced by herbivores) and the two soil activity measures. However, due to insufficient sample size, we could not include soil porosity in the SEM. The statistical analyses were performed using the R statistical software (version 4.2.2.; R Core Team 2022). Data used for creating the figures can be found in the supplementary data (Figs. 1a, 2 and 4 were created with Data 1, Figs. 1b and 3 with Data 2).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Code availability
The code is available from the corresponding author upon request.
References
Decaëns, T. Macroecological patterns in soil communities: soil community macroecology. Glob. Ecol. Biogeogr. 19, 287–302 (2010).
Bardgett, R. D. & van der Putten, W. H. Belowground biodiversity and ecosystem functioning. Nature 515, 505–511 (2014).
Oliver, M. A. & Gregory, P. J. Soil, food security and human health: a review: soil, food security and human health. Eur. J. Soil Sci. 66, 257–276 (2015).
Wall, D. H., Nielsen, U. N. & Six, J. Soil biodiversity and human health. Nature 528, 69–76 (2015).
Geisen, S., Wall, D. H. & van der Putten, W. H. Challenges and opportunities for soil biodiversity in the anthropocene. Curr. Biol. 29, R1036–R1044 (2019).
van der Heijden, M. G. A., Bardgett, R. D. & van Straalen, N. M. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 11, 296–310 (2008).
Crowther, T. W. et al. Biotic interactions mediate soil microbial feedbacks to climate change. Proc. Natl Acad. Sci. USA 112, 7033–7038 (2015).
Butenschoen, O., Scheu, S. & Eisenhauer, N. Interactive effects of warming, soil humidity and plant diversity on litter decomposition and microbial activity. Soil Biol. Biochem. 43, 1902–1907 (2011).
Riutta, T., Clack, H., Crockatt, M. & Slade, E. M. Landscape-scale implications of the edge effect on soil fauna activity in a temperate forest. Ecosystems 19, 534–544 (2016).
Sünnemann, M. et al. Combined effects of land-use type and climate change on soil microbial activity and invertebrate decomposer activity. Agric. Ecosyst. Environ. 318, 107490 (2021).
Thakur, M. P. et al. Reduced feeding activity of soil detritivores under warmer and drier conditions. Nat. Clim. Change 8, 75–78 (2018).
Gougoulias, C., Clark, J. M. & Shaw, L. J. The role of soil microbes in the global carbon cycle: tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems: role of soil microbes in global carbon cycle: carbon tracking & agro-cosystem management. J. Sci. Food Agric. 94, 2362–2371 (2014).
Lavelle, P. et al. Soil invertebrates and ecosystem services. Eur. J. Soil Biol. 42, S3–S15 (2006).
Bardgett, R. D. & Wardle, D. A. Aboveground-Belowground Linkages. Biotic Interactions, Ecosystem Processes, and Global Change. (Oxford Univ. Press, 2010).
Anderson, T. M. et al. Herbivory and eutrophication mediate grassland plant nutrient responses across a global climatic gradient. Ecology 99, 822–831 (2018).
Holt, R. D., Grover, J. & Tilman, D. Simple rules for interspecific dominance in systems with exploitative and apparent competition. Am. Nat. 144, 741–771 (1994).
Lind, E. M. et al. Increased grassland arthropod production with mammalian herbivory and eutrophication: a test of mediation pathways. Ecology 98, 3022–3033 (2017).
Borer, E. T. et al. Finding generality in ecology: a model for globally distributed experiments. Methods Ecol. Evol. 5, 65–73 (2014).
Bardgett, R. D. & Wardle, D. A. Herbivore-mediated linkages between aboveground and belowground communities. Ecology 84, 2258–2268 (2003).
Sitters, J. et al. Nutrient availability controls the impact of mammalian herbivores on soil carbon and nitrogen pools in grasslands. Glob. Change Biol. 26, 2060–2071 (2020).
Wassenaar, T. et al. Projecting land use changes in the Neotropics: the geography of pasture expansion into forest. Glob. Environ. Change 17, 86–104 (2007).
Neely, C., Bunning, S. & Wilkes, A. Review of evidence on drylands pastoral systems and climate change. (Food and Agriculture Organization of the United Nations, 2009).
Guitian, R. & Bardgett, R. D. Plant and soil microbial responses to defoliation in temperate semi-natural grassland. Plant and soil 220, 271–277 (2000).
Mikola, J. et al. Defoliation and patchy nutrient return drive grazing effects on plant and soil properties in a dairy cow pasture. Ecol. Monogr. 79, 221–244 (2009).
Risch, A. C. et al. Global impacts of fertilization and herbivore removal on soil net nitrogen mineralization are modulated by local climate and soil properties. Glob. Change Biol. 26, 7173–7185 (2020).
Augustine, D. J. & McNaughton, S. J. Interactive effects of ungulate herbivores, soil fertility, and variable rainfall on ecosystem processes in a semi-arid savanna. Ecosystems 9, 1242–1256 (2006).
Bardgett, R. D., Leemans, D. K., Cook, R. & Hobbs, P. J. Seasonality of the soil biota of grazed and ungrazed hill grasslands. Soil Biol. Biochem. 29, 1285–1294 (1997).
Bardgett, R. D. et al. Soil microbial community patterns related to the history and intensity of grazing in sub-montane ecosystems. Soil Biol. Biochem. 33, 1653–1664 (2001).
Ritchie, M. E., Tilman, D. & Knops, J. M. H. Herbivore effects on plant and nitrogen dynamics in oak savanna. Ecology 79, 165–177 (1998).
Pastor, J., Dewey, B., Naiman, R. J., McInnes, P. F. & Cohen, Y. Moose browsing and soil fertility in the boreal forests of Isle Royale National Park. Ecology 74, 467–480 (1993).
Cole, L., Buckland, S. M. & Bardgett, R. D. Influence of disturbance and nitrogen addition on plant and soil animal diversity in grassland. Soil Biol. Biochem. 40, 505–514 (2008).
King, K. L. & Hutchinson, K. J. The effects of sheep stocking intensity on the abundance and distribution of Mesofauna. Pastures J. Appl. Ecol. 13, 41 (1976).
Borer, E. T. et al. More salt, please: global patterns, responses and impacts of foliar sodium in grasslands. Ecol. Lett. 22, 1136–1144 (2019).
Galloway, J. N. et al. Transformation of the Nitrogen cycle: recent trends, questions, and potential solutions. Science 320, 889–892 (2008).
Vitousek, P. M. et al. Human alteration of the global nitrogen cycle: sources and consequences. Ecol. Appl. 7, 737–750 (1997).
Penuelas, J., Janssens, I. A., Ciais, P., Obersteiner, M. & Sardans, J. Anthropogenic global shifts in biospheric N and P concentrations and ratios and their impacts on biodiversity, ecosystem productivity, food security, and human health. Glob. Change Biol. 26, 1962–1985 (2020).
Wang, R. et al. Significant contribution of combustion-related emissions to the atmospheric phosphorus budget. Nat. Geosci. 8, 48–54 (2015).
Fay, P. A. et al. Grassland productivity limited by multiple nutrients. Nat. Plants 1, 15080 (2015).
Kaspari, M. The invisible hand of the periodic table: how micronutrients shape ecology. Annu. Rev. Ecol. Evol. Syst. 52, 199–219 (2021).
Siebert, J. et al. The effects of drought and nutrient addition on soil organisms vary across taxonomic groups, but are constant across seasons. Sci. Rep. 9, 639 (2019).
Treseder, K. K. Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol. Lett. 11, 1111–1120 (2008).
Janssens, I. A. et al. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 3, 315–322 (2010).
Ramirez, K. S., Craine, J. M. & Fierer, N. Nitrogen fertilization inhibits soil microbial respiration regardless of the form of nitrogen applied. Soil Biol. Biochem. 42, 2336–2338 (2010).
Güsewell, S. & Gessner, M. O. N: P ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct. Ecol. 23, 211–219 (2009).
Moro, H., Kunito, T., Saito, T., Yaguchi, N. & Sato, T. Soil microorganisms are less susceptible than crop plants to potassium deficiency. Arch. Agron. Soil Sci. 60, 1807–1813 (2014).
Borer, E. T. et al. Herbivores and nutrients control grassland plant diversity via light limitation. Nature 508, 517–520 (2014).
Kratz, W. The bait-lamina test: General aspects, applications and perspectives. Environ. Sci. Pollut. Res. 5, 94–96 (1998).
Scheu, S. Automated measurement of the respiratory response of soil microcompartments: Active microbial biomass in earthworm faeces. Soil Biol. Biochem. 24, 1113–1118 (1992).
Eisenhauer, N. et al. Plant diversity maintains multiple soil functions in future environments. eLife 7, e41228 (2018).
Konapala, G., Mishra, A. K., Wada, Y. & Mann, M. E. Climate change will affect global water availability through compounding changes in seasonal precipitation and evaporation. Nat. Commun. 11, 3044 (2020).
Schwarz, B. et al. Warming alters energetic structure and function but not resilience of soil food webs. Nat. Clim. Change 7, 895–900 (2017).
Bardgett, R. D. et al. Combatting global grassland degradation. Nat. Rev. Earth Environ. 2, 720–735 (2021).
Van Klink, R. et al. Effects of large herbivores on grassland arthropod diversity. Biol. Rev. 90, 347–366 (2015).
Bakker, E. S., Ritchie, M. E., Olff, H., Milchunas, D. G. & Knops, J. M. H. Herbivore impact on grassland plant diversity depends on habitat productivity and herbivore size. Ecol. Lett. 9, 780–788 (2006).
Orchard, V. A. & Cook, F. J. Relationship between soil respiration and soil moisture. Soil Biol. Biochem. 15, 447–453 (1983).
Blankinship, J. C., Niklaus, P. A. & Hungate, B. A. A meta-analysis of responses of soil biota to global change. Oecologia 165, 553–565 (2011).
Hueso, S., García, C. & Hernández, T. Severe drought conditions modify the microbial community structure, size and activity in amended and unamended soils. Soil Biol. Biochem. 50, 167–173 (2012).
Iglesias Briones, M. J., Ineson, P. & Piearce, T. G. Effects of climate change on soil fauna; responses of enchytraeids, Diptera larvae and tardigrades in a transplant experiment. Appl. Soil Ecol. 6, 117–134 (1997).
Hao, Y. & He, Z. Effects of grazing patterns on grassland biomass and soil environments in China: a meta-analysis. PLoS ONE 14, e0215223 (2019).
Andriuzzi, W. S. & Wall, D. H. Responses of belowground communities to large aboveground herbivores: meta‐analysis reveals biome‐dependent patterns and critical research gaps. Glob. Change Biol. 23, 3857–3868 (2017).
Seabloom, E. W. et al. Globally consistent response of plant microbiome diversity across hosts and continents to soil nutrients and herbivores. Nat. Commun. 14, 3516 (2023).
Smith, L. C. et al. Large‐scale drivers of relationships between soil microbial properties and organic carbon across Europe. Glob. Ecol. Biogeogr. 30, 2070–2083 (2021).
Wang, Z., Ji, L., Hou, X. & Schellenberg, M. P. Soil respiration in semiarid temperate grasslands under various land management. PLoS ONE 11, e0147987 (2016).
Cao, G. et al. Grazing intensity alters soil respiration in an alpine meadow on the Tibetan plateau. Soil Biol. Biochem. 36, 237–243 (2004).
Steinaker, D. F. & Wilson, S. D. Scale and density dependent relationships among roots, mycorrhizal fungi and collembola in grassland and forest. Oikos 117, 703–710 (2008).
Sagi, N., Grünzweig, J. M. & Hawlena, D. Burrowing detritivores regulate nutrient cycling in a desert ecosystem. Proc. R. Soc. B Biol. Sci. 286, 20191647 (2019).
Sitters, J. & Andriuzzi, W. S. The Ecology of Browsing and Grazing II. vol. 239 (Springer Int. Publ., 2019).
Cumming, D. H. M. & Cumming, G. S. Ungulate community structure and ecological processes: body size, hoof area and trampling in African savannas. Oecologia 134, 560–568 (2003).
Beylich, A., Oberholzer, H.-R., Schrader, S., Höper, H. & Wilke, B.-M. Evaluation of soil compaction effects on soil biota and soil biological processes in soils. Soil Tillage Res. 109, 133–143 (2010).
Horn, R., Domżżał, H., Słowińska-Jurkiewicz, A. & Van Ouwerkerk, C. Soil compaction processes and their effects on the structure of arable soils and the environment. Soil Tillage Res. 35, 23–36 (1995).
Richard, G., Cousin, I., Sillon, J. F., Bruand, A. & Guérif, J. Effect of compaction on the porosity of a silty soil: influence on unsaturated hydraulic properties: Soil compaction, pore geometry and hydraulic properties. Eur. J. Soil Sci. 52, 49–58 (2001).
Duffey, E. The effects of human trampling on the fauna of grassland litter. Biol. Conserv. 7, 255–274 (1975).
Chappell, H. G., Ainsworth, J. F., Cameron, R. A. D. & Redfern, M. The effect of trampling on a chalk grassland ecosystem. J. Appl. Ecol. 8, 869 (1971).
Kretzschmar, A. Burrowing ability of the earthworm Aporrectodea longa limited by soil compaction and water potential. Biol. Fertil. Soils 11, 48–51 (1991).
Borer, E. T. et al. Nutrients cause grassland biomass to outpace herbivory. Nat. Commun. 11, 6036 (2020).
Birkhofer, K. et al. Soil fauna feeding activity in temperate grassland soils increases with legume and grass species richness. Soil Biol. Biochem. 43, 2200–2207 (2011).
Siebert, J. et al. Extensive grassland-use sustains high levels of soil biological activity, but does not alleviate detrimental climate change effects. in Adv. Ecol. Res. 60, 25–58 (Elsevier, 2019).
Lin, Y. et al. Grazing intensity affected spatial patterns of vegetation and soil fertility in a desert steppe. Agric. Ecosyst. Environ. 138, 282–292 (2010).
Moreno, B., Garcia-Rodriguez, S., Cañizares, R., Castro, J. & Benítez, E. Rainfed olive farming in south-eastern Spain: long-term effect of soil management on biological indicators of soil quality. Agric. Ecosyst. Environ. 131, 333–339 (2009).
Sánchez-Moreno, S., Cano, M., López-Pérez, A. & Rey Benayas, J. M. Microfaunal soil food webs in Mediterranean semi-arid agroecosystems. Does organic management improve soil health? Appl. Soil Ecol. 125, 138–147 (2018).
Kent, A. D. & Triplett, E. W. Microbial communities and their interactions in soil and rhizosphere ecosystems. Annu. Rev. Microbiol. 56, 211–236 (2002).
Sjursen, H., Michelsen, A. & Jonasson, S. Effects of long-term soil warming and fertilisation on microarthropod abundances in three sub-arctic ecosystems. Appl. Soil Ecol. 30, 148–161 (2005).
Eisenhauer, N. et al. Plant diversity effects on soil microorganisms support the singular hypothesis. Ecology 91, 485–496 (2010).
Craven, D. et al. Plant diversity effects on grassland productivity are robust to both nutrient enrichment and drought. Philos. Trans. R. Soc. B Biol. Sci. 371, 20150277 (2016).
Gottschall, F. et al. Spatiotemporal dynamics of abiotic and biotic properties explain biodiversity–ecosystem‐functioning relationships. Ecol. Monogr. 92, e01490 (2022).
Delgado-Baquerizo, M. et al. The proportion of soil-borne pathogens increases with warming at the global scale. Nat. Clim. Change 10, 550–554 (2020).
Heintz-Buschart, A. et al. Microbial diversity-ecosystem function relationships across environmental gradients. Res. Ideas Outcomes 6, e52217 (2020).
Beaumelle, L., De Laender, F. & Eisenhauer, N. Biodiversity mediates the effects of stressors but not nutrients on litter decomposition. eLife 9, e55659 (2020).
Galantini, J. & Rosell, R. Long-term fertilization effects on soil organic matter quality and dynamics under different production systems in semiarid Pampean soils. Soil Tillage Res. 87, 72–79 (2006).
Liu, L. & Greaver, T. L. A global perspective on belowground carbon dynamics under nitrogen enrichment: belowground C dynamics under N enrichment. Ecol. Lett. 13, 819–828 (2010).
Ochoa‐Hueso, R. et al. Microbial processing of plant remains is co‐limited by multiple nutrients in global grasslands. Glob. Change Biol. 26, 4572–4582 (2020).
Hautier, Y., Niklaus, P. A. & Hector, A. Competition for light causes plant biodiversity loss after eutrophication. Science 324, 636–638 (2009).
Crawley, M. J. et al. Determinants of Species Richness in the Park Grass Experiment. Am. Nat. 165, 179–192 (2005).
Harpole, W. S. & Tilman, D. Grassland species loss resulting from reduced niche dimension. Nature 446, 791–793 (2007).
Rajaniemi, T. K. Why does fertilization reduce plant species diversity? Testing three competition-based hypotheses. J. Ecol. 90, 316–324 (2002).
DiTommaso, A. & Aarssen, L. W. Resource manipulations in natural vegetation: a review. Vegetatio 84, 9–29 (1989).
Stevens, C. J. et al. Anthropogenic nitrogen deposition predicts local grassland primary production worldwide. Ecology 96, 1459–1465 (2015).
Rocci, K. S. et al. Impacts of nutrient addition on soil carbon and nitrogen stoichiometry and stability in globally-distributed grasslands. Biogeochemistry 159, 353–370 (2022).
Joly, F.-X., Scherer-Lorenzen, M. & Hättenschwiler, S. Resolving the intricate role of climate in litter decomposition. Nat. Ecol. Evol. https://doi.org/10.1038/s41559-022-01948-z (2023).
Guerra, C. A. et al. Tracking, targeting, and conserving soil biodiversity. Science 371, 239–241 (2021).
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G. & Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005).
Van Gestel, C. A. M., Kruidenier, M. & Berg, M. P. Suitability of wheat straw decomposition, cotton strip degradation and bait-lamina feeding tests to determine soil invertebrate activity. Biol. Fertil. Soils 37, 115–123 (2003).
Hamel, C., Schellenberg, M. P., Hanson, K. & Wang, H. Evaluation of the “bait-lamina test” to assess soil microfauna feeding activity in mixed grassland. Appl. Soil Ecol. 36, 199–204 (2007).
Simpson, J. E., Slade, E., Riutta, T. & Taylor, M. E. Factors affecting soil fauna feeding activity in a fragmented lowland temperate deciduous woodland. PLoS ONE 7, e29616 (2012).
Eisenhauer, N. et al. Organic textile dye improves the visual assessment of the bait-lamina test. Appl. Soil Ecol. 82, 78–81 (2014).
Bates, D., Mächler, M., Bolker, B. & Walker, S.: Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:1406.5823 (2014).
Lüdecke, D. ggeffects: tidy data frames of marginal effects from regression models. J. Open Source Softw. 3, 772 (2018).
Cesarz, S. et al. Tree diversity effects on soil microbial biomass and respiration are context dependent across forest diversity experiments. Glob. Ecol. Biogeogr. 31, 872–885 (2022).
Lefcheck, J. S. Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573–579 (2016).
Eisenhauer, N. et al. Biodiversity-ecosystem function experiments reveal the mechanisms underlying the consequences of biodiversity change in real world ecosystems. J. Veg. Sci. 27, 1061–1070 (2016).
Acknowledgements
This work was generated using data from the Nutrient Network (http://www.nutnet.org) experiment, funded at the site-scale by individual researchers. Coordination of soil sampling was funded by a competitive WSL internal grant to A.C. Risch and S. Zimmermann. Coordination and data management for NutNet have been supported by funding to E. Borer and E. Seabloom from the National Science Foundation Research Coordination Network (NSF-DEB-1042132) and Long-Term Ecological Research (NSF-DEB-1234162 to Cedar Creek LTER) programs, and the Institute on the Environment (DG-0001-13). We also thank the Minnesota Supercomputer Institute for hosting project data and the Institute on the Environment for hosting Network meetings. J. Siebert, M. Sünnemann, and N. Eisenhauer acknowledge funding from the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig, funded by the DFG (FZT 118). M. N. Bugalho thanks the Portuguese Foundation for Science and Technology (FCT) for funding through contract DL 57/2016/CP1382/CT0030 and projects UID/BIA/50027/2013 and POCI-01-0145-FEDER-006821. M. N. Bugalho also thank Rui Alves for granting access to the study site (comp.pt) We acknowledge the Portuguese Science Foundation (FCT) for funding the research unit CEF (UIDB/00239/2020). We thank Felix Gottschall for support with Figs. 2 and 3 and especially the design of the icons.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
N.E. conceived the study; A.C.R. and N.E. developed the idea of a joint add-on project within the Nutrient Network. E.T.B. and E.W.S. coordinate the Nutrient Network. A.C.R., S.Z., and J.S. coordinated the global sampling campaign; J.D.B., L.B., D.M.B., E.T.B., M.N.B.; A.A.B.B., M.C.C., E.C.; K.F.D.; A.E.; N.H., J.M.H.K., A.S.M., R.L.M., J.L.M., S.A.P., J.N.P., E.W.S., R.S. and C.J.S. contributed data; J.S. and Y.H. analyzed the data; J.S., M.S., and N.E. wrote the manuscript with input from all authors. M.S. revised the manuscript with input from all authors (Table S5).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
We support inclusive, diverse, and equitable conduct of research.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: David Favero. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Siebert, J., Sünnemann, M., Hautier, Y. et al. Drivers of soil microbial and detritivore activity across global grasslands. Commun Biol 6, 1220 (2023). https://doi.org/10.1038/s42003-023-05607-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-05607-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.