Abstract
Local overexposure to ionizing radiation leads to chronic inflammation, vascular damage and cachexia. Here we investigate the kinetics of inflammatory cells from day (D)1 to D180 after mouse hindlimb irradiation and analyze the role of monocyte (Mo) subsets in tissue revascularization. At D1, we find that Mo and T cells are mobilized from spleen and bone marrow to the blood. New vessel formation during early phase, as demonstrated by ~1.4- and 2-fold increased angiographic score and capillary density, respectively, correlates with an increase of circulating T cells, and Mohi and type 1-like macrophages in irradiated muscle. At D90 vascular rarefaction and cachexia are observed, associated with decreased numbers of circulating Molo and Type 2-like macrophages in irradiated tissue. Moreover, CCR2- and CX3CR1-deficency negatively influences neovascularization. However adoptive transfer of Mohi enhances vessel growth. Our data demonstrate the radiation-induced dynamic inflammatory waves and the major role of inflammatory cells in neovascularization.
Similar content being viewed by others
Introduction
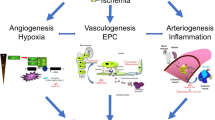
High-dose acute radiation accidents occur each year, following industrial accidents (loss of radioactive sources) or overdosage following medical applications (radiotherapy and interventional radiology procedures). The radiation injury is characterized by successive and unpredictable inflammatory waves over the first few days to years after irradiation, and these lead to the horizontal and vertical extension of tissue injury, including vascular rarefaction and muscle cachexia1. Vascular damage and rarefaction are considered to be the main cause of long-term morbimortality in patients leading to tissue ischemia after ionizing radiation exposure2. Local overexposure to ionizing radiation has severe health consequences, especially when the absorbed dose exceeds 25 Gy and leads to tissue necrosis1,3. Repair of acute skeletal muscle injury is a tightly regulated process, which mainly consists in three phases, including inflammation, regeneration, and angiogenesis4,5. In the preclinical model of total body irradiation, it has been shown that the numbers of both myonuclei and satellite cells per myofiber were decreased in a dose-dependent manner6. Moreover, a single irradiation dose of 18 Gy blocks muscle regeneration by inducing lethality of myoblasts7. Studies of muscular pathology using more than 25 Gy irradiation dose have shown morphological alterations, hemorrhage, necrosis, inflammation, fibrosis and mitochondria destruction8,9,10,11. Ischemia is the common process in both diseases radio-local injury and cardiovascular disease. In ischemic disease, insufficient organ perfusion following thrombotic vessel obstruction of the feeding artery is a major determinant of postischemic remodeling12. However, exposure of mammalian cells such as endothelial cells to ionizing radiation leads primarily to DNA damage-induced cell death13. Ischemia is characterized by vascular damage/rarefaction and inflammation resulting in fibrosis characterized by collagen-based scar14. The ischemic tissue response is based on four principal processes, vasculogenesis, angiogenesis, arteriogenesis, and collateral growth, which contribute to tissue repair and remodeling during acute and chronic ischemic vascular diseases15. These processes result from hemodynamical forces changes within the vascular wall leading to modification of the vascular homeostasis15,16.
Infiltration of inflammatory cells in hypoxic areas is a hallmark of tissue ischemia, and the respective role of distinct leukocyte subsets in postischemic neovascularization—CD4+ and CD8+ T cells17,18, NK cells19, regulatory T cells20, mast cells21, monocytes/macrophages22—is not completely understood. In particular, T lymphocytes are involved in this process, as demonstrated by the fact that nude mice, which lack all T-cell subsets, exhibit a pronounced reduction in postischemic vessel growth23. Leukocytes and monocytes trigger neovascularization through the release of several angiogenic/arteriogenic factors, including vascular endothelial growth factor (VEGF), pro-inflammatory cytokines such as tumor necrosis factor-α, interleukin (IL)-1β, and metalloproteinases24,25. In addition, the role of monocytes in the neovascularization process has been documented by different groups18,26.
Monocytes are a heterogeneous population with two major subtypes in mice: Ly6ChiLy6G-7/4hi “inflammatory” monocytes (Mohi) and Ly6CloLy6G-7/4lo “resident” monocytes (Molo) corresponding to the CD14hiCD16− and CD14loCD16+ subpopulations in humans, respectively27. Mohi express the inducible NO synthase (NOS) and pro-inflammatory cytokines, such as IL-1 and IL-12, whereas the Molo produce large amounts of arginase 1, the anti-inflammatory cytokine IL-10, and VEGF. Mohi rapidly enter sites of inflammation, while Molo enter lymphoid and non-lymphoid organs under homeostatic conditions, patrol across the vascular endothelium in a CX3CR1-dependent manner28 and favor tissue regeneration in diverse contexts29. We recently showed the central role for monocyte/macrophage activation in the orchestration of neovascularization mechanism in a mouse model of local colorectal irradiation22. In addition, in a mouse model of hindlimb ischemia, the transplantation of Mohi, but not of Molo, led to the activation of postischemic neovascularization26.
Monocyte recruitment to ischemic areas is thought to occur mainly via chemokine/chemokine receptor signaling. In particular, CCL2 and its cognate receptor CCR2, as well as fractalkine (CX3CL1) and the ligands of CX3CR1 are implicated in this context30,31,32. In fact, CCL2 is upregulated at site of collateral growth, and revascularization is markedly enhanced with CCL2 treatment33. Similarly, in ischemic hindlimb models, deficiency in CCL2 or CCR2, reduces postischemic inflammation and vessel growth25,34 and enhances gastrocnemius muscle atrophy35. Therapeutic modulation of the inflammatory response may therefore hold promise to improve reparative response for the prevention of postischemic disease15,16.
Here, using a mouse model of localized hindlimb radiation-induced injury, we analyzed the dynamics of mobilization and recruitment of innate and adaptive inflammatory cells in different tissues (bone marrow (BM), spleen, blood, and muscles) from D1 to D180 post irradiation. We showed for the first time the inflammatory waves characterizing ionizing radiation injury. These waves corresponded to the alternance between proliferation/mobilization phases observed in different tissues, such as the spleen, blood, and muscle; however, they were less pronounced in BM. We highlighted two phases after hindlimb irradiation, corresponding first to an early phase with inflammation and neovascularization correlated with the mobilization of CD4- and CD8 T cells from the spleen, infiltration in the muscle of Mohi and their differentiation into pro-inflammatory macrophages (Type 1-like macrophages/M1-like). In the late phase, we demonstrated vascular rarefaction and cachexia, which correlated with a decrease of Molo counts in blood and muscle and the differentiation of Molo into Type 2-like macrophages (M2-like) in muscle.
Finally, we investigated more precisely the role of Mohi and M1-like on neovascularization during the early phase. The deficiency on CCR2 or CX3CR1 hampered arteriogenesis mechanisms, whereas Mohi intramuscular injection in WT mice improved arteriogenesis.
Results
Localized hindlimb irradiation induces injury and rhabdomyolysis
To investigate the irradiation effect on the hindlimb, we first assessed the kinetics of evolution of the lesion by a semi-quantitative analysis of the wound extent, ulceration, moist desquamation, and limb retraction during the wound healing process each week until D180. After 14 days post irradiation, the injury score increased and peaked at D35 and then slowly decreased until D180 (Fig. 1a). This deleterious effect of irradiation was associated with cachexia/muscle wasting (rhabdomyolysis). Gastrocnemius and tibialis weight decreased progressively and significantly at D120, 150, and 180 by 2- and 1.5-fold, respectively, compared with nonirradiated group (P < 0.01, Fig. 1b).
a Kinetic evolution of injury scores mesured each week and representative photographs of radiation-induced hindlimb injuries at different time points. b Evolution of muscular weight measured on tibialis and gastrocnemius, nonirradiated (NIR) and irradiated, at days 1, 3, 5, 7, 14, 30, 60, 90, 120, 150, and 180 post irradiation. *P < 0.05; **P < 0.01; ***P < 0.001 vs. NIR; n = 8 animals /time point, ANOVA +/− SEM.
Localized hindlimb irradiation induces proliferation, mobilization, and recruitment of innate inflammatory cells
BM and spleen are known to release inflammatory cells after injury and contribute to the inflammatory process after irradiation22. CD11b+ leukocytes corresponding to neutrophils and monocytes were mobilized from the BM or spleen to the blood and then infiltrated in irradiated muscle (Fig. 2a, b). We did not see any accumulation of monocytes in muscle, suggesting they differentiated in macrophages36. Nonirradiated (NIR) corresponds to littermates euthanized at different time points and showed comparable results in term of the number of inflammatory cells in tissues (blood, spleen, bone marrow, and muscles) at all times.
a–d Quantitative analysis (left) and representative flow cytometry gating (right) of cell counts for neutrophils (CD45 + CD11b+Ly6G+7/4hi, green line), Mohi (CD45 + CD11b+Ly6G–7/4hi, red line) and Molo (CD45 + CD11b+Ly6G–7/4lo, blue line) (gated on CD45+ and CD11b+ cells) in bone marrow, spleen, blood, and muscle, respectively. e Quantitative analysis (left) and representative flow cytometry gating (right) of cell counts for DC (CD45 + CD11b+CD11c+MHCII+), M1-like Mɸ (CD45+CD11b-F4/80+CD64+MHCII+) and M2-like Mɸ (CD45+CD11b-F4/80+CD64+MHCII−) in muscle, nonirradiated (NIR) and irradiated, at days 1, 3, 5, 7, 14, 30, 60, 90, 120, 150, and 180 post irradiation. *P < 0.05; **P < 0.01; ***P < 0.001 vs. NIR; n = 8–10 animals /time point, ANOVA +/− SEM.
In BM, neutrophils (CD11b+Ly6G+7/4hi) significantly proliferated at D1, D150, and D180 (P < 0.001) compared to NIR (Fig. 2a). Mohi (CD11b+Ly6G-7/4hi) proliferated at D14 and D180 (P < 0.05) and Molo (CD11b+Ly6G-7/4lo) at D150 and D180 (P < 0.001) respectively compared to NIR (Fig. 2a). Interestingly, at D5, neutrophils, Mohi and Molo were depleted compared to NIR and then proliferated to return to basal level at D7 until D90. In addition, neutrophils, Mohi, and Molo numbers increased in the spleen at D14 and D150 (P < 0.001, Fig. 2b).
In the blood, neutrophils, Mohi, and Molo counts increased from D1 until the end of the experiment (P < 0.001, Fig. 2c).
In muscle, Mohi, Molo, and neutrophils were recruited and peaked at D1, D5, and D60 and then returned to basal level at D3, D30, and D120 (Fig. 2d). M2-like peaked at D1 (P < 0.01, Fig. 2e), whereas dendritic cells peaked at D60 (P < 0.01, Fig. 2e) after muscle irradiation. We also noticed an increase, though not significant, of M1-like in muscle at D60.
These results suggest that, after muscle irradiation, innate inflammatory cells are recruited early and massively from spleen and BM into the muscle. Moreover, these results show the time-recurring, consecutive inflammatory waves following radiation injury, corresponding to alternate phases of innate inflammatory cells proliferation in BM and spleen (Fig. 2a, b) and mobilization through the blood (Fig. 2c), and then their infiltration in irradiated muscle (Fig. 2d, e).
Localized hindlimb irradiation induces the mobilization and recruitment of adaptive inflammatory cells
We previously showed, in a mouse model of colorectal injury, that after irradiation, T lymphocytes were recruited to the injured tissue22. Here we found that, in our model, CD4 and CD8 T cells were similarly mobilized from the spleen and showed a strong significant decrease from D1 (P < 0.001, Fig. 3a) until D90. Afterward, T cells proliferated and almost reached basal level at D120, and then T-cell numbers decreased again at D150 and D180 (P < 0.001, Fig. 3a) compared to NIR. In blood, T-cell numbers increased and peaked at D30 and D60 (P < 0.001, Fig. 3b). In muscle, T cells were recruited and peaked at D1 and D30 (P < 0.001, Fig. 3c).
a–c Quantitative analysis (left) and representative flow cytometry gating (right) of cell counts for CD4 (CD45+CD3+CD4+) and CD8 (CD45+CD3+CD8+) in spleen, blood, and muscle, nonirradiated (NIR) and irradiated, at days 1, 3, 5, 7, 14, 30, 60, 90, 120, 150, and 180 post irradiation. *P < 0.05; **P < 0.01; ***P < 0.001 vs. NIR; n = 8–10 animals/time point, ANOVA +/− SEM.
These results show that localized hindlimb irradiation induced a depletion of T-cell numbers in the spleen, suggesting their mobilization and recruitment from the spleen to the irradiated muscle. Similar to innate cells, we observed consecutive waves of T-cell mobilization and recruitment in blood and muscles. However, we did not observe such waves in the spleen, probably because the T cells were immediately mobilized.
Localized hindlimb irradiation induces cytokines and chemokines expression in muscle
CCL2 and CX3CL1 are known to contribute to neovascularization via the attraction and retention of monocytes and T cells in ischemic leg26,37.
mRNA expression of CCL2 significantly increased by 11- and 14-fold at D14 and D30, respectively, in irradiated muscle compared to NIR (Fig. 4a). Similarly, mRNA expression of CX3CL1 significantly increased by 3.7-fold at D30 and 7.7-fold at D90 in irradiated muscle compared to NIR tissue (Fig. 4b).
a Quantitative evaluation of CCL2 and CX3CL1 mRNA in muscle. b, c Quantitative evaluation (left-side panels) and representative photomicrographs (right-side panels) of CD68-positive cells (b, indicated by arrows) or CD3-positive cells (c, indicated by arrows) per mm2 in the nonirradiated (NIR) and irradiated muscle. Results were expressed as percentages relative to NIR, set to 100% and irradiated muscle was normalized to the NIR muscle at days 1, 3, 5, 7, 14, 30, 60, 90, 120, 150, and 180 post irradiation, scale bar: 100 µm. *P < 0.05; **P < 0.01; ***P < 0.001 vs. NIR; n = 6 animals/time point, ANOVA +/− SEM.
Chemokine upregulation was associated with macrophage and lymphocyte infiltration in muscle. At D7 and D14 after irradiation, the number of CD68-positive macrophages increased by 3.5- and 4-fold (P < 0.001), respectively, and decreased at D30, compared to NIR tissue. The number of CD3-positive lymphocytes increased by 39-fold at D7, 24–fold at D14, and 18-fold at D30 (Fig. 4b, c and supplementary Fig. 1).
These results show that localized irradiation results in the upregulation of chemokines such as CCL2 and CX3CL1 and inflammatory cell recruitment.
Localized hindlimb irradiation induces transient neovascularization process in muscle
We next analyzed the pro-angiogenic actors eNOS in muscle post irradiation. eNOS mRNA levels in irradiated muscle were upregulated by 2.8-, 2.6-, 2.5-, and 2.3-fold, respectively, at D7, D14, D30, and D90 compared to NIR (Fig. 5a). Accordingly, eNOS protein levels were also significantly upregulated at D30 by 2.3-fold compared to NIR (P < 0.001, Fig. 5b).
a–d Quantification of eNOS mRNA and protein levels in muscle. e, f Quantitative evaluation and representative photomicrographs of microangiography (c; vessels appear in white) and capillary density (d; capillaries appear in red, arrows indicate examples of isolectin B4-positive capillaries, scale bar: 100 µm). Results were expressed as percentages relative to nonirradiated (NIR), set to 100%, and irradiated muscle was normalized to NIR muscle, at days 1, 3, 5, 7, 14, 30, 60, 90, 120, 150, and 180 post irradiation. *P < 0.05; **P < 0.01; ***P < 0.001 vs. NIR; n = 5–8 animals /time point, ANOVA +/− SEM.
Furthermore, we evaluated the neovascularization process following irradiation in the muscle by using independent and complementary microangiography and immunohistochemistry approaches. The angiographic score was significantly increased by 1.4-fold (P < 0.01) at D14 compared with the NIR group. In addition, capillary density was upregulated at D7 and D14 by 1.7- (P < 0.05) and twofold (P < 0.001), respectively, compared with the NIR group (Fig. 5c, d). Moreover, by the Bayesian latent variable approach38, we analyzed the correlation between neovascularization process and mobilization/recruitment of inflammatory cells during the early phase (D0-D30) and late phase (D60-D180). From D1 to D30 post irradiation, we found a significant correlation between new vessel formation and mobilization of CD4/CD8 cells (P < 0.01 both) from the spleen and their increase in the blood (P < 0.05 both) (Table 1). In the same way, the increase of Mohi infiltrated numbers and their differentiation into M1-like in irradiated muscle was associated with the neovascularization (P < 0.01 both) (Table 1).
However, at D60, angiographic score returned to the basal level, and then decreased significantly at D90, D120 and D150 (P < 0.01) compared with the NIR group (Fig. 5c). Furthermore, at D30, capillary density returned to the NIR level and significantly decreased from D60 to D180 (P < 0.05) compared to the NIR group (Fig. 5d). More importantly, from D60 to D180, we demonstrated by Bayesian approach that the decrease of Molo counts in blood (P < 0.05) and in muscle (P < 0.001), as well as their differentiation in M2-like in muscle (P < 0.05) correlated with this process of vascular rarefaction (Table 1).
Differential role of chemokine receptor in the control of circulating monocyte levels after irradiation
In order to directly address the role of monocytes/macrophages in the neovascularization process after hindlimb irradiation, we investigated more precisely the role of Mohi and Molo by using CCR2- or CX3CR1-deficient mice. In Ccr2−/− mice, circulating Mohi (P < 0.001, Fig. 6a) and Molo (P < 0.01, Fig. 6b) levels were significantly reduced when compared with wild-type mice at D3 following irradiation. Moreover, Lu et al. showed that mobilization of monocytes (Mohi and Molo), but not lymphocytes or neutrophils, was impaired from BM to blood and from blood to injured muscle in Ccr2−/− mice suggesting that the low level of monocyte analyzed in blood was not due to hindlimb irradiation39. Similarly, we also demonstrated the low number of macrophages in CCR2−/− muscle compared to WT mice at 10 days post irradiation (P < 0.05; Fig. 6d). Next, we sought to evaluate the impact of the CCR2 and CX3CR1 signaling pathways on postirradiation vessel growth. Ten days after irradiation, angiographic score was reduced in CCR2- and CX3CR1-deficient mice compared to their wild-type counterparts (P < 0.001, Fig. 6e).
a–c Quantitative analysis of cell counts for Mohi, Molo, and neutrophils in blood in WT mice or CCR2- and CX3CR1-deficient mice. **P < 0.01; ***P < 0.001 vs. irradiated WT, n = 3–4 animals/time point. d Quantitative analysis of CD68+ macrophages infiltration from blood to injured muscles at D10 post irradiation in WT mice or CCR2- and CX3CR1-deficient mice, n = 5 animals/time point. e Quantitative evaluation and representative photomicrographs of microangiography in WT mice or CCR2- and CX3CR1-deficient mice 10 days after hindlimb irradiation. f Quantitative evaluation and representative photomicrographs of microangiography 10 days after hindlimb irradiation in animals intramuscular injected with PBS, 105 Mohi or 105 Molo. *P < 0.05 vs. PBS injected mice. Results were expressed as percentage relative to nonirradiated (NIR), set to 100% and irradiated muscle was normalized to NIR muscle, n = 5–12 animals/time point, ANOVA +/− SEM.
As expected, our results clearly show that disruption of the CCL2/CCR2 and CX3CL1/CX3CR1 pathways hampers postischemic vessel growth.
Mohi promote neovascularization after hindlimb irradiation
Because Molo monocytes are scarce in the BM and difficult to collect in sufficient amounts from blood, we used splenic Mohi and Molo as surrogates for circulating monocyte subsets. In addition, it was shown that blood and spleen monocyte subsets are almost identical in transcriptome and function40. On the day after hindlimb irradiation, WT C57BL/6 mice received intramuscular injection of PBS, 105 Mohi, or 105 Molo. 10 days following irradiation, Mohi transfer improved angiographic score (P < 0.5, Fig. 6f) compared with PBS-treated mice. In contrast, injection of Molo did not improve angiographic score.
These findings clearly identify a central role for Mohi activation in the orchestration of vascular inflammation and the promotion of neovascularization in the acute phase after hindlimb irradiation.
Discussion
Local overexposure to ionizing radiation has severe health consequences, especially when the absorbed dose exceeds 25 Gy and leads to tissue necrosis1. The radiation injury is characterized by successive and unpredictable inflammatory waves over the first few days to weeks after irradiation, and these lead to surface and depth extension of tissue injury including vascular rarefaction and muscle cachexia1. In this report, we showed, for the first time, the dynamic inflammatory waves characterizing radiation-induced injury. In the spleen and the bone marrow, these waves corresponded to alternate phases between cell proliferation and mobilization through the blood and then their infiltration in irradiated muscles. Theses waves were much more pronounced for neutrophils, Mohi and then Molo mainly in the spleen and muscle. However, in the blood, the number of innate circulating cells were increased from D1 to D180 post irradiation, whereas in irradiated muscle, their number was quite low suggesting an accumulation in the blood. In addition, T cells waves were observed in blood and muscle. Many studies have already shown the mobilization and recruitment of inflammatory cells after colorectal localized irradiation22, femoral ischemia or myocardial infarction20,24,41. However, compared to the classical cardiovascular ischemic model14, the numbers of M2-like macrophages in irradiated muscle were increased only at D1. We have several hypotheses that could explain this observation, including (i) the proliferation of M2 resident cells in the tissue, (ii) the defect in M2 egress (migration capability)42 or (iii) Resistance of radio-induced apoptosis of M2 cells process in the muscle.
Several studies have previously shown that irradiated ischemic endothelium displays pro-inflammatory properties. Endothelial cells express chemokines such as CCL2 and CX3CL1, leading to the recruitment and infiltration of inflammatory cells through their receptors CCR2 or CX3CR1 to improve new vessel formation26,30. We showed that irradiated tissue expresses, during the early phase (D0-D30 post-IR), pro-angiogenic (eNOS) and pro-arteriogenic factors (CX3CL1, CCL2) to induce mobilization of pro-inflammatory CD4-, CD8 T cells, and Mohi from the spleen and BM through the blood and their recruitment and infiltration in irradiated tissue leading to the differentiation of Mohi into M1-like macrophages. Afterward, we observed a late phase (D60-D180 post-IR) characterized by a correlation between the reduced anti-inflammatory Molo recruitment and their differentiation into M2-like, vascular rarefaction, and cachexia. Although, at D90 post irradiation, we could detect an increase of CX3CL1 and eNOS mRNA expression, we did not observe similar increase of the corresponding proteins, which is in agreement with vascular rarefaction and cachexia.
These results are in line with the literature concerning the role of CCL2 and CX3CL1 in participating to the recruitment of inflammatory cells and improving neovascularization. Hence after femoral artery occlusion, local infusion or overexpression of CCL2 increased circulating monocyte numbers, and their accumulation was shown to support collateral and peripheral conductance, and neovascularization26,31. CX3CL1-deficient mice contained significantly fewer macrophages than controls in atherogenesis model43. These data add to accumulating evidence showing that monocytes/chemokines pathways play a central role in regulating angiogenesis. Moreover, T lymphocytes are also involved in this process since nude mice, which lack all T-cell subsets, exhibit a pronounced reduction in postischemic vessel growth23. Furthermore, CD4 and CD8 T-cell subsets have been suggested to play a role in vascular remodeling. Indeed, CD4- and CD8-deficient mice exhibit a significant reduction in postischemic vessel growth17,18.
Next, we showed that monocytes contributed to neovascularization process after a localized hindlimb irradiation by using CCR2- and CX3CR1-deficient mice. The deletion of CCR2 or CX3CR1 reduced the number of circulating monocytes and decreased angiographic score as previously reported. CCR2 and CX3CR1 have been shown to control monocyte recruitment to ischemic tissues30,44. CCR2 is mainly involved in the regulation of circulating Mohi levels. Of interest, lack of CCR2 specifically abrogates Mohi infiltration in the ischemic myocardium29,40. Also, it has been shown that CCR2 expression by BM cells, but not by injured muscle tissue cells, is required to mount an inflammatory response following acute skeletal muscle injury45 to allow subsequent muscle capillary arterialization46. Mohi facilitate arteriogenesis and angiogenesis by producing growth factors35,47,48,49. Moreover, CX3CR1 signaling has been shown to promote the survival of monocytes that are short-lived cells50: endogenous CX3CR1 signaling might be required for the survival of newly mobilized BM-originating monocytes. This is supported by our observation of a lower number of Molo monocytes in CX3CR1-/- mice compared to WT animals. CX3CR1-deficient mice revealed adequate monocyte recruitment and revascularization for skin repair and wound angiogenesis; however, the myeloid cell response and magnitude of neovascularization were dampened compared with wild-type mice51. In addition, in atherosclerosis, many studies have shown that CX3CL1/CX3CR1 signaling was implicated in angiogenesis during plaque microvessel formation44,52,53.
Finally, we highlighted a major role of Mohi monocytes in postischemic vessel growth. Muscular administration of Mohi enhanced arteriogenesis, whereas Molo injection had no effect on this process. We show that adoptive transfer of the inflammatory subset of monocytes early after induction of hindlimb ischemia markedly enhances reperfusion following ischemia. It has been demonstrated, in a rabbit model of acute femoral artery ligation, that the collateral artery growth depends directly on the number of circulating monocytes25. Moreover, the distinct pro-arteriogenic potential might result from the differential expression of angiogenic factors such as MMP-926,54 through the release of extracellular matrix-bound VEGF55. In myocardial infarction, a selective depletion of M2 macrophages had a catastrophic prognosis with an increase of frequent cardiac rupture. The tissue repair was entirely rescued by an external supply of M2-like macrophages56. These results are in accordance with the role of M1-like and M2-like cells in tissue repair that corresponds to their sequential and complementary functions, in that M1-like macrophages initiate the healing process by stimulating angiogenesis38,57 while M2-like cells promote stabilization of angiogenesis and tissue maturation by supporting the increase of regenerating fibers diameter58 during skeletal muscle regeneration in mice.
Thus, monocyte/macrophage could be used as biomarkers to evaluate and manage the irradiated tissue to offer personalized regenerative medicine treatment to the patient. Infiltration of M1-/M2-like macrophages could be investigated by three different methods. The most suitable for the evaluation of macrophage infiltration is flow cytometry with antibody mixtures, but this approach depends on the amount of harvested tissue. Thus, PCR and immunohistochemical staining might be used to analyze expression of cytokines or inflammatory cells.
In summary, we showed that inflammatory cells play a major role after hindlimb irradiation to allow the neovascularization process and maintain blood vessels to limit cachexia development.
Methods
Animals, irradiation
All animal procedures performed were approved by the institutional animal experimentation and ethics committee (C2EA) at the Institute of Radiation Protection and Nuclear Safety (IRSN). Experiments were conducted according to the French veterinary guidelines and those formulated by the European Community for experimental animal use (APAFIS#12903-2018010418086132 v1 and APAFIS #19137-2019021313569870 v1). Male C57Bl/6 mice (8 weeks old) were used as experimental animals (Janvier, France). Eight-week-old male CCR2−/−, CR3CR1−/− and their wild-type (WT) C57BL/6 littermates were purchased from the Jackson Laboratories. All animals were kept under stable microenvironment conditions (22 ± 1 °C), with alternating 12 h light and dark cycles and received standard laboratory food and water. Mice were anesthetized with isoflurane inhalation (2%) and their right hindlimb was exposed to a single dose of 80 Gy irradiation using 10-MV X-rays at 2.6 Gy/min on an irradiation field of 2*24 cm (Elekta Synergy Platform, Elekta SAS, Boulogne-Billancourt, France).
Scoring injury
The kinetics of the evolution of the lesion was assessed by a semi-quantitative analysis. A score between 0 (normal) and 1 (max injury) was assigned to each clinical sign, including wound extent, ulceration, moist desquamation and limb retraction resulting in a total injury scoring up to 5 during the wound development and healing process each week post irradiation.
Immunohistochemistry
Gastrocnemius were excised, rinsed in PBS, and frozen in liquid nitrogen. Tissues were cut using a cryostat into 7-μm-thick sections.
Macrophages and CD3-positive cells were visualized after CD68 antibody (1/250, Biorad) or CD3 staining (1/250, DAKO) followed by staining with a donkey 488-AF–anti-rat or anti-rabbit IgG (1/250, Jackson ImmunoResearch) respectively. CD68- and CD3-positive cells were counted in randomly chosen fields with the use of Image J software (NIH). Sections were stained with hematoxylin and eosin (H&E) to analyze muscle structure.
Analysis of neovascularization
After irradiation, neovascularisation was evaluated by two different methods as previously59.
Microangiography
At the time of sacrifice, mice were anesthetized (Alfaxan (80 mg/kg body weight) and Xylazine (10 mg/kg body weight), and longitudinal laparotomy was performed to introduce a polyethylene catheter into the abdominal aorta and inject contrast medium (barium sulfate, 1 g/mL). Angiography of hindlimbs was then performed, and images (two per animal) were acquired with the use of a high-definition digital X-ray transducer. Images were assembled to obtain a complete view of the hindlimbs. The number of pixels occupied by vessels was measured in the quantification area with the use of Primed angio software (Trophy System, Paris, France). The area of quantification was limited by the placement of the iliac, the knee, the edge of the femur, and the external limit of the leg. The results were then expressed as a ratio of irradiated to nonirradiated leg.
Capillary density measurement
Gastrocnemius muscle was excised from the right (irradiated limb) and the left (NIR limb), and frozen in O.C.T. compound (Sakura Finetek France, Villeneuve d’ASCQ, France) for cryosectioning. Frozen muscle sections (7 µm) were stained using a DyLight 594 Labeled GSLI-isolectin B4 (Vector Laboratories, dilution 1:200) to identify capillaries. The number of capillaries per mm2 in muscle area was determined.
Flow cytometry
Mice were euthanized by cervical dislocation after sedation with isoflurane inhalation (4%) on days 1, 3, 5, 7, 14, 30, 60, 90, 120, 150, or 180 after hindlimb irradiation (n = 8–10 mice per time point). Peripheral blood was drawn via inferior vena cava puncture with heparin solution. Whole blood was lysed after immunofluorescence staining using the BD FACS lysing solution (BD Biosciences), and total blood leukocyte numbers were determined using Kova slides. Bone marrow cells were drawn from the femur and filtered through a 40-μm nylon mesh (BD Biosciences). Spleens were collected, and gently passed through a 40-μm nylon mesh (BD Biosciences). For both splenocytes and bone marrow-derived cells, the cell suspension was centrifuged at 400×g for 10 min at 4 °C. Red blood cells were lysed using red blood cell lysing buffer (Sigma-Aldrich) and splenocytes and bone marrow cells were washed with PBS. Nonirradiated and irradiated muscles were collected, minced with fine scissors minced with fine scissors, and gently passed through the Bel-Art Scienceware 12-well tissue disaggregator (ThermoFisher Scientific). Cells were then filtered through a nylon mesh (40 μm) and centrifuged (10 min, 400×g, 4 °C).
Cells isolated from the tissue of interest were incubated in the dark at 4 °C for 30 min with the following antibody mix. For detection of inflammatory cells, total cells were gated on AF700-conjugated anti-CD45, FITC-conjugated anti-CD11b (ThermoFisher Scientific), APC-conjugated anti-Ly-6B.2 (clone 7/4, Biorad), PE-conjugated anti-Ly6G, PB-conjugated anti-CD64, APC-conjugated anti-F4/80, PerCP5.5-conjugated anti-MHCII (BD Biosciences), FITC-conjugated anti-CD4 (clone RM4-5; eBioscience), PercpCy5-conjugated anti-CD8 (clone 53-6.7, BD Pharmingen), APC-conjugated anti-CD3 (clone 17A2, eBioscience), PE-Cyanine7-conjugated anti-CD11c (cloneN418, eBioscience) during 30 min at 4 °C.
We used different antibody mixes in different tissues as follows:
-
A.
Bone marrow: mix 1: Mo: FITC-conjugated anti-CD11b, APC-conjugated anti-Ly-6B.2, PE-conjugated anti-Ly6G; mix 2: lymphocytes: AF700-conjugated anti-CD45, FITC-conjugated anti-CD4, PercpCy5-conjugated anti-CD8, APC-conjugated anti-CD3,
-
B.
Spleen: mix 1: Mo: FITC-conjugated anti-CD11b, APC-conjugated anti-Ly-6B.2, PE-conjugated anti-Ly6G; mix 2: lymphocytes: AF700-conjugated anti-CD45, FITC-conjugated anti-CD4, PercpCy5-conjugated anti-CD8, APC-conjugated anti-CD3,
-
C.
Blood: mix 1: Mo: FITC-conjugated anti-CD11b, APC-conjugated anti-Ly-6B.2, PE-conjugated anti-Ly6G; mix 2: lymphocytes: AF700-conjugated anti-CD45, FITC-conjugated anti-CD4, PercpCy5-conjugated anti-CD8, APC-conjugated anti-CD3,
-
D.
Muscle: mix 1: Mo: AF700-conjugated anti-CD45, FITC-conjugated anti-CD11b, APC-conjugated anti-Ly-6B.2, PE-conjugated anti-Ly6G; mix 2: lymphocytes: AF700-conjugated anti-CD45, FITC-conjugated anti-CD4, PercpCy5-conjugated anti-CD8, APC-conjugated anti-CD3, mix 3: Macrophages: AF700-conjugated anti-CD45, FITC-conjugated anti-CD11b, PE-conjugated anti-Ly6G, PB-conjugated anti-CD64, APC-conjugated anti-F4/80, PerCP5.5-conjugated anti-MHCII, PE-Cyanine7-conjugated anti-CD11c Mohi (CD11b+Ly6G–7/4hi), Molo (CD11b+Ly6G–7/4lo), M1-like cells (CD11b+Ly6G-F4/80+CD64+MHCII+) and M2-like cells (CD11b+Ly6G-F4/80+CD64+MHCII-), DC (CD11c+MHCII+), CD4 (CD3+CD4+) and CD8 (CD3+CD4+).
The total number of cells was then normalized to muscle weight. Cells were analyzed using a flow cytometer (Canto II, BD Biosciences).
Real-time quantitative polymerase chain reaction
Total RNA was extracted from frozen muscle samples with Trizol reagent according to the manufacturer’s instructions (Invitrogen, France). After quantification on a NanoDrop ND-1000 apparatus (NanoDrop Technologies, Rockland, DE), reverse transcription was performed using the QuantiTect Reverse Transription (Qiagen) according to the manufacturer’s instructions. PCR was performed with the ABI PRISM 7900 using Power SYBR PCR Master Mix (Bioline). Ct for mouse GAPDH was used to normalize samples amplification. The following oligonucleotides (Applied Biosystems) served as primers: GAPDH forward: 5′-CGTCCCGTAGACAAAATGGTGAA-3′, reverse: 5′-GCCGTGAGTGGAGTCATACTGGAACA-3′; eNOS forward: 5′-CGCCCACCCAGGAGAGATCCAC-3′, reverse 5′-GCATCGGCAGCCAAACACCAAAGT-3′; CCL-2 forward: 5′-CCCCACTCACCTGCTGCTA-3′, reverse 5′-TTACGGGTCAACTTCACATTCAAA-3′; CX3CL1 forward: 5′-GTGGCTTTGCTCATCCGCTATCAG-3′, reverse: 5′-CACATTGTCCACCCGCTTCTCA-3′.
Western blot
Preparation of protein extracts was performed. Briefly, to prepare total protein, tibialis anterior muscles from irradiated and nonirradiated hindlimbs were homogenized in RIPA buffer (50 mM Tris HCl pH7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% deoxycholate, 0.1% SDS with protease and phosphatase inhibitors). Proteins were resolved in gradient denaturing gel electrophoresis and blotted onto 0.2μm nitrocellulose sheets (Biorad, Marnes la Coquette, France). Antibodies against eNOS (1:1000; BD Biosciences) were used for immunoblotting. As a protein loading control, membranes were stripped and incubated with a monoclonal antibody directed against GAPDH (1:10,000, Abcam) and specific chemiluminescent signal was detected.
Monocyte adoptive transfer experiment
For the adoptive transfer experiment, spleens from 8-week-old C57BL/6 mice were mechanically dissociated on a 40-mm cell strainer. Splenocytes stained with AF700-conjugated anti-CD45, FITC-conjugated anti-CD11b, APC-conjugated anti-Ly-6B.2, Pacific Blue-conjugated anti-Ly6G and PE-conjugated anti-NK1.1. CD11b+Ly6G-NK1.1- 7/4hi and CD11b + Ly6G2NK1.1- 7/4lo were then selected using a FACS BD InFlux. Purity for both subset was 99%. Before injection, cells were counted by trypan blue exclusion. In total, 105 Mohi monocytes or 105 Molo monocytes were then intramuscular injected to C57BL/6 recipients the next day after hindlimb irradiation.
Statistic and reproducibility
Results were expressed as ± SEM. One-way analysis of variance (ANOVA) was used to compare each parameter. Post hoc Bonferroni’s t test comparisons were then performed to identify which group differences account for the significant overall ANOVA. A value of P < 0.05 was considered significant.
The investigation of the statistical association between infiltration of inflammatory cells and leukocyte subsets in one hand with postischemic neovascularization in other hand is challenging since these two measures were performed on different animal cohorts. This makes the classical statistical approaches inefficient since predictive and response variables are not observed on the same subject. The Bayesian latent variable model60 offers the possibility to include priors on the distribution of the inflammatory cells and leukocyte subset counts to estimate their correlation with the vessel growth. The model fitting was performed using JAGS software via Markov chain Monte Carlos. All P values in the different statistical analysis sections were corrected for multiple testing using Benjamini and Hochberg procedure.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data that supported the findings of this study are included within this paper and its Supplementary Information files and Supplementary Data 1.
References
Zhao, W. & Robbins, M. E. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr. Med. Chem. 16, 130–143 (2009).
Milliat, F., Francois, A., Tamarat, R. & Benderitter, M. Role of endothelium in radiation-induced normal tissue damages. Annales de cardiologie et d’angeiologie 57, 139–148 (2008).
Reyes, E. H. et al. Medical response to radiological accidents in Latin America and international assistance. Radiat. Res. 185, 359–365 (2016).
Tidball, J. G. Inflammatory processes in muscle injury and repair. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R345–R353 (2005).
Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 9, 653–660 (2003).
Masuda, S. et al. Time- and dose-dependent effects of total-body ionizing radiation on muscle stem cells. Physiol. Rep. 3, https://doi.org/10.14814/phy2.12377 (2015).
Quinlan, J. G. et al. Regeneration-blocked mdx muscle: in vivo model for testing treatments. Muscle Nerve 20, 1016–1023 (1997).
Hsu, H. Y., Chai, C. Y. & Lee, M. S. Radiation-induced muscle damage in rats after fractionated high-dose irradiation. Radiat. Res. 149, 482–486 (1998).
Gerstner, H. B., Lewis, R. B. & Richey, E. O. Early effects of high intensity x-radiation on skeletal muscle. J. Gen. Physiol. 37, 445–459 (1954).
Persons, C. C., Hermens, A. F., Franken, N. A. & Wondergem, J. Muscle wasting after radiotherapy in young and adult rats. Oncol. Rep. 8, 1117–1122 (2001).
Sun, W. et al. Adipose-derived stem cells alleviate radiation-induced muscular fibrosis by suppressing the expression of TGF-beta1. Stem Cells Int. 2016, 5638204 (2016).
Silvestre, J. S. Pro-angiogenic cell-based therapy for the treatment of ischemic cardiovascular diseases. Thromb. Res. 130, S90–S94 (2012).
Billis, W., Fuks, Z. & Kolesnick, R. Signaling in and regulation of ionizing radiation-induced apoptosis in endothelial cells. Recent Prog. Horm. Res. 53, 85–92 (1998).
Zouggari, Y. et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat. Med. 19, 1273–1280 (2013).
Loinard, C. et al. Inhibition of prolyl hydroxylase domain proteins promotes therapeutic revascularization. Circulation 120, 50–59 (2009).
Loinard, C. et al. HuMSC-EV induce monocyte/macrophage mobilization to orchestrate neovascularization in wound healing process following radiation injury. Cell Death Discov. 9, 38 (2023).
Stabile, E. et al. Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation 108, 205–210 (2003).
Stabile, E. et al. CD8+ T lymphocytes regulate the arteriogenic response to ischemia by infiltrating the site of collateral vessel development and recruiting CD4+ mononuclear cells through the expression of interleukin-16. Circulation 113, 118–124 (2006).
van Weel, V. et al. Natural killer cells and CD4+ T-cells modulate collateral artery development. Arterioscler. Thromb. Vasc. Biol. 27, 2310–2318 (2007).
Zouggari, Y. et al. Regulatory T cells modulate postischemic neovascularization. Circulation 120, 1415–1425 (2009).
Heissig, B. et al. Low-dose irradiation promotes tissue revascularization through VEGF release from mast cells and MMP-9-mediated progenitor cell mobilization. J. Exp. Med. 202, 739–750 (2005).
Loinard, C., Vilar, J., Milliat, F., Levy, B. & Benderitter, M. Monocytes/macrophages mobilization orchestrate neovascularization after localized colorectal irradiation. Radiat. Res. 187, 549–561 (2017).
Couffinhal, T. et al. Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in ApoE-/- mice. Circulation 99, 3188–3198 (1999).
Sunderkotter, C., Goebeler, M., Schulze-Osthoff, K., Bhardwaj, R. & Sorg, C. Macrophage-derived angiogenesis factors. Pharm. Ther. 51, 195–216 (1991).
Heil, M. et al. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking CC-chemokine receptor-2. Circ. Res. 94, 671–677 (2004).
Cochain, C. et al. Regulation of monocyte subset systemic levels by distinct chemokine receptors controls post-ischaemic neovascularization. Cardiovasc. Res. 88, 186–195 (2010).
Auffray, C., Sieweke, M. H. & Geissmann, F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 27, 669–692 (2009).
Auffray, C. et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317, 666–670 (2007).
Nahrendorf, M. et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 (2007).
Heil, M. & Schaper, W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis). Circ. Res. 95, 449–458 (2004).
Ito, W. D. et al. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ. Res. 80, 829–837 (1997).
Swirski, F. K. et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Investig. 117, 195–205 (2007).
Engel, D. R. et al. CCR2 mediates homeostatic and inflammatory release of Gr1(high) monocytes from the bone marrow, but is dispensable for bladder infiltration in bacterial urinary tract infection. J. Immunol. 181, 5579–5586 (2008).
Waeckel, L. et al. Tetrapeptide AcSDKP induces postischemic neovascularization through monocyte chemoattractant protein-1 signaling. Arterioscler. Thromb. Vasc. Biol. 26, 773–779 (2006).
Heil, M. et al. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am. J. Physiol. Heart Circ. Physiol. 283, H2411–H2419 (2002).
Auffray, C. et al. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J. Exp. Med. 206, 595–606 (2009).
Imai, T. et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 91, 521–530 (1997).
Spiller, K. L. et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 35, 4477–4488 (2014).
Lu, H. et al. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J. 25, 358–369 (2011).
Swirski, F. K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 (2009).
Engel, W. K. & Hawley, R. J. Focal lesions of muscle in peripheral vascular disease. J. Neurol. 215, 161–168 (1977).
Koscso, B. et al. Gut-resident CX3CR1(hi) macrophages induce tertiary lymphoid structures and IgA response in situ. Sci. Immunol. 5, https://doi.org/10.1126/sciimmunol.aax0062 (2020).
Teupser, D. et al. Major reduction of atherosclerosis in fractalkine (CX3CL1)-deficient mice is at the brachiocephalic artery, not the aortic root. Proc. Natl Acad. Sci. USA 101, 17795–17800 (2004).
Combadiere, C. et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J. Clin. Investig. 117, 2920–2928 (2007).
Sun, D. et al. Bone marrow-derived cell regulation of skeletal muscle regeneration. FASEB J. 23, 382–395 (2009).
Nickerson, M. M. et al. Capillary arterialization requires the bone-marrow-derived cell (BMC)-specific expression of chemokine (C-C motif) receptor-2, but BMCs do not transdifferentiate into microvascular smooth muscle. Angiogenesis 12, 355–363 (2009).
Arras, M. et al. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J. Clin. Investig. 101, 40–50 (1998).
Kinnaird, T. et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 109, 1543–1549 (2004).
Rehman, J., Li, J., Orschell, C. M. & March, K. L. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107, 1164–1169 (2003).
Landsman, L. et al. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood 113, 963–972 (2009).
Khan, B., Rangasamy, S., McGuire, P. G. & Howdieshell, T. R. The role of monocyte subsets in myocutaneous revascularization. J. Surg. Res. 183, 963–975 (2013).
Pamukcu, B., Lip, G. Y., Devitt, A., Griffiths, H. & Shantsila, E. The role of monocytes in atherosclerotic coronary artery disease. Ann. Med. 42, 394–403 (2010).
Lee, S. J. et al. Fractalkine stimulates angiogenesis by activating the Raf-1/MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways. Am. J. Physiol. Heart Circ. Physiol. 291, H2836–H2846 (2006).
Johnson, C., Sung, H. J., Lessner, S. M., Fini, M. E. & Galis, Z. S. Matrix metalloproteinase-9 is required for adequate angiogenic revascularization of ischemic tissues: potential role in capillary branching. Circ. Res. 94, 262–268 (2004).
Bergers, G. et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2, 737–744 (2000).
Shiraishi, M. et al. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J. Clin. Investig. 126, 2151–2166 (2016).
Willenborg, S. et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood 120, 613–625 (2012).
Arnold, L. et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069 (2007).
Silvestre, J. S. et al. Proangiogenic effect of angiotensin-converting enzyme inhibition is mediated by the bradykinin B(2) receptor pathway. Circ. Res. 89, 678–683 (2001).
Song, X. Y. & Lee, S. Y. Basic and Advanced Bayesian Structural Equation Modeling: With Applications in the Medical and Behavioral Sciences (Wiley, 2012a).
Acknowledgements
Dr Celine Loinard is supported by Institut de Radioprotection et de Sûreté Nucléaire (IRSN), Fontenay-aux-Roses, France.
Author information
Authors and Affiliations
Contributions
C.L and R.T. conceived the study, designed the experiments, and interpreted the data. C.L., M.A.B., B.L., S.F., and J.B. performed the experiments and analyzed the data. C.L. and R.T. wrote the manuscript with final approval from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Jemond Dalli and Karli Montague-Cardoso. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Loinard, C., Benadjaoud, M.A., Lhomme, B. et al. Inflammatory cells dynamics control neovascularization and tissue healing after localized radiation induced injury in mice. Commun Biol 6, 571 (2023). https://doi.org/10.1038/s42003-023-04939-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-04939-3
This article is cited by
-
microRNA blood signature for localized radiation injury
Scientific Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.