Abstract
To perform natural transformation, one of the three main Horizontal Gene Transfer mechanisms, bacteria need to enter a physiological differentiated state called genetic competence. Interestingly, new bacteria displaying such aptitude are often discovered, and one of the latest is the human pathogen Staphylococcus aureus.
Here, we show an optimized protocol, based on planktonic cells cultures, leading to a large percentage of the population activating the development of competence and a significant improvement of S. aureus natural transformation efficiencies. Taking advantage of these conditions, we perform transcriptomics analyses to characterize the regulon of each central competence regulator. SigH and ComK1 are both found essential for activating natural transformation genes but also important for activation or repression of peripheral functions. Even though ComK2 is not found important for the control of transformation genes, its regulon shows an important overlap with that of SigH and ComK1. Finally, we propose that microaerobic conditions, sensed by the SrrAB two-component system, are key to activate competence in S. aureus.
Similar content being viewed by others
Introduction
A highly adaptive commensal organism, such as Staphylococcus aureus, possesses an array of genes that allows the bacterium to grow, infect and survive in a wide variety of ecological niches. The anterior nares are generally considered the native ecological niche of S. aureus, though the bacterium can be isolated from other areas of the human body, including the skin, the axillae, the groin, and the gastrointestinal tract1. While colonization is typically not harmful, S. aureus may breach innate host defenses and gain access to deeper tissues, causing a variety of superficial and invasive infections2. Furthermore, as a facultative anaerobe, S. aureus has the ability to grow and thwart the host immune system in the presence or absence of oxygen. This capacity is particularly important for S. aureus as its environment is known to be or become anaerobic during the course of an infection3,4.
In addition to these remarkable adaptive powers, S. aureus also became one of the most feared pathogens in the hospital because of the widespread emergence of antibiotic multi-resistant strains5,6. Horizontal gene transfer (HGT) of antibiotic resistance genes from other S. aureus strains or even from other genera was though for years to be exclusively mediated through conjugation and transduction7. However, a few years ago, the demonstration that S. aureus is capable of becoming naturally competent for genetic transformation8 changed our way of apprehending HGT in this important human pathogen.
Competence is a physiological adaptation that some bacterial species develop in response to various environmental signals9. In response to these stimuli, bacterial cells trigger signal transduction pathways, ultimately activating central competence regulators. All these steps are controlled by the so-called early competence genes. Interestingly, central competence regulators have been identified in several model organisms as transcriptional activators10,11 or alternative sigma factors12. Once activated, they initiate the expression of the late competence genes, among which are found all the genes essential for genetic transformation.
Importantly, three potential central competence regulators have been identified in S. aureus: the alternative sigma factor, SigH13, and two transcriptional regulators, ComK1 and ComK214. In addition, it has been shown that S. aureus is able to naturally induce competence in a chemically defined medium called CS28. The authors detected a maximum of 1.6% of competence-inducing cells after 8 h of growth in the CS2 medium, leading to transformation efficiencies that barely reached the detection limit (around 10−10) for a wild-type strain8.
In this study, our main objective was to characterize the development of competence in the human pathogen S. aureus. Our goal was to investigate all the steps involved, from the environmental stimuli sensed by the cells and the signal transduction pathways to the central competence regulators and their regulons. To reach such goals, we first developed a protocol to optimize the development of competence and genetic transformation in S. aureus planktonic cultures. Then, using various reporter strains and global transcriptomic analysis, we analyzed the role of the three central regulators during the development of competence in S. aureus. We particularly showed that while SigH and ComK1 are both essential for the expression of the genes involved in genetic transformation, the three regulators (SigH, ComK1, and ComK2) are all required for the full development of the competence transcriptional program. Finally, we propose that oxygen limitation, sensed by the SrrAB two-component system (TCS), probably activating SigH, controls the development of competence in the human pathogen S. aureus.
Results
An optimized protocol to induce competence for genetic transformation in S. aureus with high efficiency
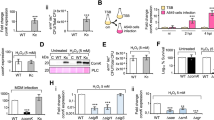
We first decided to optimize the protocol published by Morikawa and his colleagues back in 20128 (see Material and methods for details). In order to compare our results, we used the same reporter strain, expressing the gfp gene under the control of the comG late competence operon’s promoter (PcomG-gfp)8. Briefly, the reporter strain was first streaked on a BHI agarose plate. Isolated colonies are then used to inoculate a pre-culture in the BHI medium. This pre-culture was then quickly stopped in exponential growth, centrifuged, washed, and used to inoculate a fresh culture in a CS2 medium. From this initial culture in fresh CS2, tenfold serial dilutions were made in closed Falcon tubes. Finally, cell density (OD600 nm) and the percentage of competent GFP-expressing cells were measured through flow cytometry in each diluted culture every 30 min. Interestingly, Fig. 1a, b demonstrates how our optimized protocol was able to induce the development of competence in up to 70% of the population in a given experiment (in reality, statistical analysis evaluated this percentage at 52 ± 15% of the population, n = 12). This percentage, more than ten times higher than in the literature, was previously calculated through the detection of GFP-expressing cells by microscopy8. Therefore, we verified that measurements performed by flow cytometry would not introduce any bias and provide similar results to microscopy (Supplementary Fig. 1).
a Growth of a wild-type strain expressing gfp under the control of the comG promoter (St29) in CS2 medium, between 16 and 21.5 h. Dilutions from 10−2 to 10−5 are shown. The 10−2 dilution was already in the stationary phase, while the 10−3 dilution was entering the stationary phase after 16 h of growth. The 10−4 and 10−5 dilutions were, respectively, in the late and early exponential phase at the beginning of the experiment. All the dilutions reached a similar final OD between 2.4 and 2.6. b The percentage of competent cells was measured using flow cytometry by analyzing the percentage of cells expressing GFP under the control of the comG promoter (PcomG). In each diluted culture presented in panel a), the percentage of competent GFP-expressing cells increased in the late exponential phase and reached a maximum at the entry in the stationary phase. This graph shows a representative experiment that has been repeated three times (biological replicates, see Supplementary Fig. 2a). c Transformation efficiencies of wild type (N315ex w/o Phi), comGA (St137), sigH (St45), comK1 (St37), comK2 (St38), and srrA (St117) mutant strains using plasmidic (pCN34, KanR, white bars) or chromosomal DNA (gray bars) (see Material and methods for details). Results are presented as mean ± SD. For each strain, the experiment was repeated at least five times (biological replicates). Individual experiments are shown as blue circles. d Transformation efficiencies of laboratory strains (N315, RN4220, and USA300) and MRSA (NL10, NL27) or MSSA (NL36) clinical isolates using chromosomal DNA (gray bars) (see material and methods for details). Results are presented as mean ± SD. For each strain, the experiment was repeated at least five times (biological replicates). Individual experiments are shown as blue circles.
We then confirmed that our optimized protocol, associated with improved development of competence, also led to higher transformation efficiencies. Interestingly, under laboratory conditions, an N315 wild-type strain reached a transformation efficiency around 1.5 × 10−7 (±0.6 × 10−8) with a replicative plasmid as donor DNA (Fig. 1c). Such a result is 1000 times higher than what has been previously published8. Importantly, the transformation efficiency even reached 4.2 × 10−6 (±4.1 × 10−6) when we used an S. aureus strain’ chromosome (harboring an antibiotic marker, see Material and methods) as exogenous DNA (Fig. 1c). To definitely confirm that the colonies growing in our assays were real transformants, we also showed that no transformation events could be detected using a strain in which comGA, an essential gene for genetic transformation15, was deleted (Fig. 1c).
Importantly, the protocol described here and optimized for the N315 strain also leads other laboratory strains (RN4220 and USA300) and clinical isolates (NL10, NL27, and NL36) to genetic transformation (Fig. 1d). Interestingly, RN4220 displayed very high transformation efficiencies (reaching 9.7 × 10−5 ±5.9 × 10−5) while USA300 (7 × 10−8 ±6.9 × 10−8) and the clinical isolates (NL10, 1.2 × 10−7 ±3.79 × 10−7; NL27, 1.6 × 10−7 ±4.8 × 10−7 and NL36, 1.2 × 10−7 ±7.9 × 10−7) showed lower numbers than N315. These results clearly demonstrate the robustness of our protocol, but also that further optimization might be required for new strains.
Competence develops at a specific cell density and growth phase in CS2
Figure 1 clearly shows how competence was induced in each diluted culture using our optimized protocol. Each additional dilution was characterized by a 2-h delay in growth (Fig. 1a). Indeed, the 10−5 dilution needs more time to reach the stationary phase than the 10−4 dilution, which needs more time than the 10−3 dilution. Accordingly, the development of competence was also delayed in each consecutive diluted culture (Fig. 1b). However, GFP-expressing competent cells always appeared when the cultures approached OD = 2 and reached a maximum once each culture entered the stationary phase (Fig. 1a, b).
To further verify the existence of a correlation between competence development and cell density, we repeated this experiment three times (Supplementary Fig. 2a). This correlation clearly showed that competence development in S. aureus grown in CS2 reached a maximum as the cultures entered the stationary phase with an OD600nm around 2.4 (in fact between 2.2 and 2.6) (Supplementary Fig. 2a). High cell density, sensed by bacterial cells through quorum sensing (QS), has been proposed and verified in several model organisms as important stress, inducing competence16,17,18. Therefore, we then hypothesized that a QS system could be involved in the development of competence in S. aureus. Interestingly, two QS systems have been identified in S. aureus (Agr and Lux19). Thus, we finally investigated the impact of agrA (encoding the Agr system transcriptional regulator,19) and luxS (encoding the Lux system regulator,19) gene deletion on the development of competence. Surprisingly, none of these genes were found involved in the induction of the comG operon’s expression (Supplementary Fig. 2b). This result seemed surprising, especially when compared to recent data where the deletion of agrAC decreased competence by a threefold factor20. Even though the protocols used in this study are different from ours, further investigations will be required to understand why QS does not seem involved in all conditions.
PcomG, Pssb, PcomC, and PcomF are not controlled by the same regulators
As mentioned in the introduction, three potential competence regulators have been identified in the literature. Even though SigH has been shown to be important to activate the expression from PcomG8, no role has been clearly assigned to ComK1 and ComK2. Here, using our optimized protocol, we decided to test the effect of sigH, comK1, and comK2 deletion on the expression from four promoters: PcomG (a promoter only activated by SigH overexpression,8,14), Pssb (a promoter that could only be activated by ComK1 over-expression,14), PcomC and PcomF (two promoters that could not be activated by any regulator14).
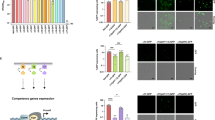
As expected, in a wild-type background, the four promoters were found activated with an average percentage of GFP-expressing cells of 51.87% for PcomG, 77.26% for Pssb, 38.32% for PcomC and 38.56% for PcomF (Fig. 2 and Supplementary Fig. 3). When sigH was deleted, only the expression from PcomG was lost (Fig. 2a). This result confirms that SigH does not control the expression of all the genetic transformation genes and that at least one additional regulator must be involved. Interestingly, when comK1 was inactivated, expression from all the promoters was abolished (Fig. 2a–d). Therefore, ComK1 is essential, alongside SigH, for the comG operon expression but is also absolutely required, alone, for ssb and comC expression. Interestingly, comF regulation seemed intermediate, as deletion of comK1 abolishes comF expression while the absence of sigH decreases it by almost a twofold factor (Fig. 2d). Finally, in a comk2 mutant, no effect could be detected for all promoters.
Percentage of the population expressing GFP under control of PcomG (St29, St51, St40, and St 41) (a), Pssb (St50, St61, St64, and St67) (b), PcomC (St48, St60, St63, and St66) (c), and PcomF (St233, St235, St234, and St236) (d) in a wild-type background or in the absence of sigH, comK1, or comK2 was determined after 21 h of growth in CS2 medium. Results are presented as mean ± SD. Each experiment has been repeated at least five times (biological replicates). Individual experiments are shown as blue circles.
SigH and ComK1 control genetic transformation, not ComK2
To complete our results (Fig. 2) and exhaustively characterize the SigH, ComK1, and ComK2 regulons, we then performed a global transcriptional analysis through RNA-sequencing by comparing the impact of the deletion of the genes encoding these three individual regulators on the competence transcriptional program.
First, we focused on the genes involved in natural genetic transformation (Fig. 3 and Supplementary Table 1). The results previously obtained were confirmed despite some small differences (discussed in Supplementary Note 1). Overall, SigH and ComK1 were both found essential for the expression of most genetic transformation genes, with the exception of ssb, which is only controlled by ComK1. Again, no role could be attributed to ComK2. Finally, we verified the transformation efficiency of strains were the genes encoding the central competence regulators were individually deleted (Fig. 1c). Expectedly, only the absence of sigH or comK1 abolished genetic transformation, while a comK2 mutant displayed a transformation efficiency comparable to that of a wild type strain (Fig. 1c).
Late competence genes for which the expression is induced (>2-fold, a) or repressed (>2-fold, b). Each colored circle represents a regulon, for which the expression is activated or inhibited by SigH (in blue), ComK1 (in yellow), ComK2 (in green), or by the three (outside the black circle). The number of genes in each category is shown inside the circles or at the intersection between circles when the same gene is controlled by more than one regulator. The table below shows the total number of genes for which the expression is induced or repressed by a twofold, threefold, or fivefold factor. Activated (c) and repressed (d) late competence genes (>2-fold) are also presented per function. The number of genes controlled by each central competence regulator (SigH in blue, ComK1 in yellow, and ComK2 in green) is shown inside the circles or at the intersection between circles when the same gene is controlled by more than one regulator.
SigH, ComK1, and ComK2 are all required for the full development of competence
In other model organisms, the competence transcriptional program always encompasses more genes than just the genes involved in genetic transformation21,22,23,24. It is clearly also the case in S. aureus. Indeed, in comparison to the sigH, comK1, and comK2 mutant strains, 213/73/95 genes were respectively found overexpressed by at least a twofold factor in the wild-type strain (Fig. 3a). Furthermore, if we considered higher over-expression cutoffs, the number of genes found overexpressed were still important, with 84/38/53 genes overexpressed by a three-fold factor and even 35/23/31 genes overexpressed by a five-fold factor. The numbers found here, especially for the three-fold expression factor, are quite similar to what has been described in another model organisms21,22,23,24, revealing a true core set of around 100 late competence genes in S. aureus.
We then analyzed the functions associated to the genes induced during S. aureus competence transcriptional program (Fig. 3c, Supplementary Tables 2–4). In addition to the natural genetic transformation genes, we found genes involved in (i) the regulation of the stress response (mainly but not exclusively controlled by ComK2), (ii) genes encoding multiple putative Toxin/Antitoxin systems25 (controlled by the three regulators), (iii) genes involved in the amino and nucleic acids metabolism (controlled by SigH and ComK2) and (iv) genes involved in iron transport (mainly but not exclusively controlled by SigH). Importantly, our results clearly established that even though only SigH and ComK1 are essential for the expression of the genetic transformation genes, the central competence regulators (i.e., SigH, ComK1, and ComK2) are all absolutely required for the full development of the competence transcriptional program in S. aureus.
Competence development is associated with virulence inhibition
Our global transcriptional analysis also revealed that the expression of numerous genes was inhibited during the development of competence in S. aureus. Indeed, we found that a total of 399 genes were inhibited (>2-fold) in the wild-type strain compared to the sigH, comK1, or comK2 individual mutant strains (Fig. 3b). This represents more than 15% of all the genes present in the N315 S. aureus’ genome.
Interestingly, more than 10% of the repressed genes (37 to be exact) are directly involved in virulence or virulence regulation (Fig. 3d, Supplementary Table 5). Among these genes were found many well-characterized virulence factors, including genes encoding for the capsule (CapA-O), serine proteases (SplA-F, SspA-C), intercellular adhesins (IcaAB), exoproteins (Hlg, Coa), surface proteins (ClfAB, fnb, FnbB, geh, sdrCD, Spa) and a lipase (Geh). In addition, the expression of an important TCS known to drive the expression of over 20 virulence factors in vivo, saeRS26, was found repressed during competence. Even though all the central competence regulators were found involved in virulence repression, SigH and ComK2 played a major role with an important overlap between the genes they each repress (Fig. 3d, Supplementary Table 5). Unfortunately, a comparison of wild type and sigH or comK2 mutant exoproteomes did not show any significant difference in the number of virulence factors (Supplementary Fig. 4). Importantly, in our cultures, two populations co-exist, competent and non-competent cells, each representing 50% of the total population. Therefore, as the non-competent cells still produce virulence factors, the effect of the inhibition associated with the competent cells on the total amount of virulence factors produced in the culture would be underestimated. Alternatively, the cells could have been collected at a time point that might not be appropriate to observe the effect on the exoproteome.
Oxygen limitation is an important environmental signal to induce the development of competence
Finally, competence development in S. aureus has been demonstrated in different conditions associated to different oxygen availability8. Thus, we wondered if oxygen-sensing TCS present in S. aureus (i.e., SrrAB27, NreBC28, and AirRS29) were involved during the early competence regulation steps in the course of planktonic growth in the CS2 medium. To test such a hypothesis, we compared the expression from the comG promoter in wild-type and srrA, nreC, and airR mutant strains. In this experiment, deletion of nreC and airR did not affect the expression from the comG promoter (Fig. 4a). However, when srrA was absent, comG expression was decreased by a sevenfold factor (Fig. 4a). Since both SigH and ComK1 are involved in the comG operon expression (Fig. 2a), we then decided to determine which central competence regulator was under the control of SrrAB. As ssb expression was found only controlled by ComK1 (Fig. 2b), we finally tested the effect of srrA deletion on its expression. In the absence of srrA, the expression of ssb was not affected (Fig. 4b). In addition, deletion of both sigH and srrA had the same impact on the ssb expression as the individual sigH and srrA mutant strains (Fig. 4b). Altogether, these results suggested that SrrAB might activate SigH to control the expression from the comG promoter. Moreover, the implication of SrrAB in the regulatory pathways leading to competence development and genetic transformation implies that the absence of srrA must have an effect on S. aureus genetic transformation efficiencies. Indeed, when the srrA gene was deleted, a 15 and 19-fold decrease (respectively obtained using plasmid or chromosomal DNA) in S. aureus genetic transformation efficiency was observed (Fig. 1c).
a Percentage of cells expressing GFP from PcomG in wild type (St29), ssrA (St145), nreC (St158), and airR (St177) mutant strains. Results are presented as mean ± SD. Each experiment has been repeated at least five times (biological replicates). Individual experiments are shown as blue circles. b Percentage of cells expressing GFP from Pssb in wild type (St50), srrA (St147), sigH (St61), and srrA/sigH double mutant (St252) strains. Results are presented as mean ± SD. Each experiment has been repeated at least five times (biological replicates). Individual experiments are shown as blue circles.
Oxygen-sensing TCS allows S. aureus to survey and respond to microaerobic or anaerobic conditions. In particular, SrrAB has been shown to be a global regulator of virulence factors under low-oxygen conditions27. It is, therefore, tempting to speculate that during S. aureus growth in the CS2 medium, the oxygen concentration dropped in the culture. To test this hypothesis, we finally measured the concentration of dissolved oxygen throughout growth in CS2 (Fig. 5 and Supplementary Fig. 5). Interestingly, as the OD started to increase, the oxygen concentration quickly dropped. Indeed, while the oxygen concentration stayed constant at 21% for the first 8 h, it then decreased down to 0.27% during the next 6 h, while the culture’s OD only reached 0.5. A few minutes after the oxygen concentration reached its lowest point, the culture reproducibly paused for roughly 1 h, potentially to adapt to these new low oxygen conditions (Fig. 5). Finally, following the drop in the oxygen concentration and the pause in growth, GFP-expressing competent cells started to emerge (Fig. 5). Therefore, we can confidently propose that using our optimized protocol, growth in CS2 medium is associated to a quick limitation in the oxygen availability, leading to microaerobic conditions sensed by SrrAB which in turn activates SigH for the induction of competence in S. aureus.
A wild-type strain (St29) expressing GFP under the control of PcomG was grown in a CS2 medium for 24 h. When growth became detectable, the oxygen concentration, the percentage of GFP-expressing cells, and OD600 nm were measured every 30 min. As the OD increased, the concentration of oxygen quickly dropped from 21% to 0.27%, while the OD only reached 0.5. Importantly, our assay allows us to affirm that complete anaerobic conditions are not reached as our sensors are able to measure with confidence oxygen concentrations that are ten times lower. A few minutes later, the growth reproducibly marked a pause, probably necessary for the cells to adapt to low oxygen conditions, which in turn induced an increase in the percentage of competent GFP-expressing cells. This Figure shows a representative experiment that has been reproduced five times (see Supplementary Fig. 5 for replicates).
Discussion
Previously, the work of Fagerlund and her colleagues14 clearly established that the central competence regulator’s individual or combined over-expression was not enough to induce the full development of competence in S. aureus. In this study, we present an optimized protocol allowing the optimum induction of genetic competence in S. aureus. The such protocol was essential to lead the genetic study proposed here. Importantly, the resulting transformation efficiencies detected with several laboratory strains and clinical isolates ultimately demonstrate the true potential of this HGT mechanism to modulate S. aureus genetic plasticity and antibiotic resistance genes acquisition in vivo.
In addition, we also established that three central competence regulators are essential for a complete development of the competence transcriptional program in S. aureus. While the importance of SigH was already known, we demonstrate for the first time the essentiality of ComK1 for the expression of the genes involved in genetic transformation. Importantly, we also reveal how ComK2 is involved, alongside SigH and ComK1, in the complete development of the competence transcriptional program (Fig. 6). Indeed, our global transcriptomic study clearly shows that in addition to genetic transformation, numerous other functions are also induced during competence, a feature shared with other model organisms. It will be important to investigate in the future how these other biological processes (i.e., stress response30, amino31 and nucleic32 acid metabolism or toxin/antitoxin systems33,34) participate to the establishment of the competence environmental adaptation.
Three central competence regulators have been identified, namely SigH, ComK1, and ComK28,14. All three regulators are essential for a complete development of the competence transcriptional program. While SigH and ComK1 are absolutely required for the expression of genetic transformation genes, ComK2 (alongside SigH and ComK1) is also essential for the induction of additional cellular functions (i.e., stress response, toxin/antitoxin systems, amino and nucleic acid metabolism, iron transport…). In addition, the development of competence is characterized by the inhibition of virulence, mainly controlled by SigH and ComK2. Finally, we have shown that the natural development of competence is induced as the oxygen concentration in the culture is drastically decreased. This oxygen rarefaction is probably sensed by the SrrAB two-component system, which in turn activates the central competence regulator SigH.
The presence and involvement of three central competence regulators in S. aureus, each probably induced by distinct signal transduction pathways, is a striking feature. The overlapping of the SigH, ComK1, and ComK2 regulons is another one, even though such overlapping has already been described in Vibrio cholerae, in which two central competence regulators have been identified35. One hypothesis could be that S. aureus needs multiple regulatory pathways in order to integrate a wider range of cues to decide whether or not to become competent for genetic transformation. According to this hypothesis, several environmental signals should be present concomitantly to allow the optimum development of genetic competence in S. aureus. On the other hand, such complex global regulation provides multiple targets to modulate competence development when S. aureus is not in these optimal conditions. Interestingly, ComK2, which does not control the expression of the genetic transformation genes in microaerobic environments, could become involved in response to different specific environmental signals, complexifying the regulation for the development of competence in S. aureus.
Furthermore, it is interesting to mention that the regulation of competence development in S. aureus shares traits with several important historical model organisms: S. pneumoniae and its alternative sigma factor (ComX), phylogenetically close to SigH13, B. subtilis and its central competence regulator, ComK, homologous to ComK1 and ComK214 and V. cholerae through the presence of several central competence regulators18. Thus, it would be interesting and probably important to test in the future if other regulatory features present in these historical model organisms could also be used by S. aureus to control or modulate the development of competence.
Finally, we demonstrate how oxygen limitation, leading to microaerobic conditions sensed by the SrrAB TCS, controls the development of competence in S. aureus. Since our cultures are performed in closed tubes, similarly to what was previously published36, we think that growing S. aureus in CS2 leads to rapid consumption of dissolved oxygen that cannot be replaced by atmospheric oxygen. Results from the literature already indicated that S. aureus had the ability to induce competence under different oxygen concentrations8,20. Induction of competence under anaerobic conditions8, during biofilm formation20 or in response to ROS37, further reinforces this idea. We also proposed that SrrAB could activate SigH (through transcription, translation, or stability) in response to variations in the environmental oxygen concentration. Additional work will be required to fully understand how a decrease in the oxygen concentration activates, directly or indirectly, SigH through SrrAB. Interestingly, the work of Cordero and colleagues shows how competence development is associated with dramatic changes in carbon metabolism37. It would be, therefore, important to test if the regulation of the cellular physiology, in response to oxygen limitations, could be involved in the development of competence in S. aureus.
Importantly, microaerobic conditions are often encountered by S. aureus in vivo. Indeed, during an infection, energy-consuming activated neutrophils trigger oxygen deficiency, while macrophages, dendritic cells, and T cells induce inflammation, altering blood flow to tissues and reducing oxygen levels dramatically38. In addition, oxygen-restricted microenvironments are formed during biofilm-associated infections39 or abscesses40. Therefore, during the course of an infection, S. aureus is often exposed to the environmental conditions described in this manuscript and leading to the development of competence for genetic transformation, reinforcing the true potential of this HGT mode in vivo. However, our global transcriptomic analysis also revealed that the transcription of numerous virulence genes was repressed during the natural development of competence (Fig. 6). Therefore, as an in vivo model, we can propose that during the course of an infection, S. aureus cells induce the expression of the virulence regulon, which would ultimately lead to a local oxygen deficiency. A restricted percentage of the population then senses this environmental signal and, in response, induces competence for genetic transformation in a restricted part of the population. Ultimately, the competent S. aureus cells repress virulence and promote HGT, while the rest of the population continues to promote the infection.
Materials and methods
Bacterial strains and culture conditions
N3158, RN422041, and USA30042 S. aureus strains, as well as clinical isolates43 used in this project, are all listed in Supplementary Table 6. S. aureus strains were grown in BHI medium (Becton, Dickinson, and Company) or a completely synthetic medium called CS28, depending on the experiment. When necessary, antibiotics were used to select specific events (Kan, 200 µg/mL; Cm, 10 µg/mL).
Optimized dilution protocol to naturally induce competence in S. aureus
Cells were isolated from −80 °C stock on the BHI plate. Four clones were inoculated in 10 mL of BHI and incubated at 37 °C with shaking at 180 rpm until OD reached 2.5. This pre-culture was then centrifuged, washed in fresh CS2 medium, and used to inoculate 10 mL of fresh CS2 medium (OD = 0.5) in closed 50 mL Falcon tubes. From this initial CS2 culture, serial 10-fold dilutions were performed to generate 10−1, 10−2, 10−3, 10−4, and 10−5 cultures closed 50 mL Falcon tubes, and more if needed. Several tubes for each dilution were prepared in order to take individual samples along growth (i.e., each Falcon tube was only opened once for one sample). Dilutions were finally incubated overnight at 37 °C with shaking at 120 rpm. Cells were collected when the OD was between 2 and 2.2 (GFP reporter strains, Figs. 2 and 3) or throughout growth (GFP reporter strains, Fig. 1, oxygen measurements, Fig. 4).
Construction of GFP reporter plasmids and strains
Promoters of interest were inserted in the pRIT-GFP plasmid44 using the Gibson assembly method. The promoters and the pRIT-GFP plasmid were first amplified with dedicated primers. All the oligonucleotides used in this study are listed in Supplementary Table 7. In every primer’s tail, we designed 25–50 bp homologous overlap regions between the extremities of each promoter and the pRIT-GFP linear plasmid. The promoters and the pRIT-GFP plasmid were amplified using the Phusion High-Fidelity DNA Polymerase (purchased from Thermo Scientific). All the PCR fragments were then purified using a standard commercial silicon column (PCR cleanup kit, Macherey-Nagen) and verified by gel electrophoresis for the absence of non-specific or minor PCR fragments.
Gibson assembly master mix was prepared by adding 320 µL of 5× ISO buffer (25% w/v PEG-8000; 500 mM Tris-HCl, pH 7.5; 50 mM MgCl2; 50 mM DTT; 5 mM NAD; 1 mM each dNTP), 0.64 µL 10 U/µL T5 exonuclease, 20 µL of 2 U/µL Phusion polymerase, 160 µL of 40 U/µL Taq DNA ligase and 699.36 µL of water (all reagents were purchased from New England Biolabs). Five microlitres of the two DNA fragments mixture containing 100 ng of linear pRIT plasmid and a 3-fold excess of inserts were added to 15 µL of Gibson assembly master mix. The reaction tubes were then incubated at 50 °C for 1 hr. Finally, 1 µL of the assembly reaction was transformed into IM08B electro-competent Escherichia coli cells. Transformed E. coli cells were incubated at 37 °C on LB agar with 100 µg/mL ampicillin.
In order to select the transformants containing the expected plasmid, colony PCR was performed. The resulting plasmids were extracted from positive transformants (overnight cultures) and purified using a commercial kit (NucleoSpin Plasmid extraction kit, Macherey-Nagen). All the plasmids were verified by sequencing (GATC company). Finally, in order to obtain the final reporter strains, S. aureus electrocompetent cells were transformed with each constructed plasmid.
Construction of S. aureus deletion mutants
In order to investigate the role of the main genes predicted to be involved in the regulation of competence development in S. aureus, listed in Table S1, allelic replacement constructs were cloned into the temperature sensitive pIMAY plasmid44. All the primers used for cloning in the present study are listed in Supplementary Table 7. Fragments corresponding to 1 kbp flanking regions of the genes to delete were amplified from S. aureus N315 genomic DNA using primers flanked by restriction enzyme sites compatible with the multiple cloning site (MCS) of pIMAY. The upstream or downstream regions were digested using the two chosen restriction enzymes and ligated into the pIMAY, opened with the same enzymes. The resulting plasmids were electro-transformed into the IM08B E. coli strain. Colony PCR was then used to verify the structure of the plasmids present in the transformants. Plasmids were purified from the positive colonies using a commercial kit (NucleoSpin Plasmid extraction kit, Macherey-Nagen). After verifying the sequence of the plasmid (GATC company), S. aureus N315ex woϕ strain was transformed by electroporation and plated on BHI agar supplemented with chloramphenicol (10 mg/mL) and incubated at 28 °C.
In order to allow pIMAY integration into the chromosome, a single colony from the transformation plate was resuspended in 200 µL of BHI. The suspension was diluted 10-fold down to 10−3, and 100 µL of each dilution was spread on BHI supplemented with chloramphenicol (10 mg/mL) and incubated at 37 °C overnight. The next day, colonies were subsequently streaked in the same conditions. Meanwhile, colony PCR analysis was performed to check the absence of extrachromosomal pIMAY and whether plasmid integration had occurred in the upstream or downstream region.
Based on the colony PCR results, an overnight culture in BHI at 28 °C without chloramphenicol was performed. The overnight culture was then diluted 10-fold down to 10−7. Hundred microlitres of 10−4 to 10−7 dilutions were plated onto BHI containing 1 µg/mL anhydrotetracycline (aTc). The plates were incubated at 28 °C for 2–3 days. Colonies were then patched on BHI (without antibiotic) and BHI supplemented with chloramphenicol (10 mg/mL) plates and grown at 37 °C overnight. Chloramphenicol-sensitive colonies were screened by colony PCR to identify clones containing the desired mutation. Mutant strains were finally verified by PCR and DNA sequencing.
Flow cytometry to determine the percentage of the population expressing GFP
Following growth in CS2, 500 µL of cells were harvested by centrifugation at 11,000g for 1 min. Pellets were resuspended in 500 µL of cold 70% ethanol and incubated on ice for 20 min in order to fix the cells. Then, S. aureus cells were resuspended in 500 µL of PBS (pH 7,4) after centrifugation at 11,000g for 1 min. Finally, the percentage of the population expressing GFP was evaluated by Flow cytometry (Cytoflex top-bench cytometer, Beckman-Coulter). Following Forward- and Side-scatter detection to identify individual cells, a 488 nm laser was used to distinguish GFP-expressing competent cells by comparison with the auto-fluorescence of a strain that did not express GFP (St12) (see Supplementary Fig. 1a).
It is important to mention that GFP is a very stable protein. Therefore, once the maximum percentage of competent cells was reached, this number stayed constant for hours. This feature does not mean that competence stays ‘open’ for hours but rather that once the maximum is reached, no new competent cells appear.
Microscopy to determine the percentage of GFP-expressing cells
Following growth in CS2, cells were harvested and treated as explained above (see flow cytometry). Fluorescence images of cells were taken using a confocal laser-scanning microscope (ImagerieGif platform). GFP was excited at 488 nm using the blue laser, and fluorescence images were collected using the green channel. Images were reconstituted using the ImageJ software.
Genetic transformation of competent S. aureus cells
Wild-type strain (St12) as well as comGA (St137), comK1 (St37), comK2 (St38), and sigH (St45) mutant strains were first grown to competence using our optimized dilution protocol. We chose to perform the transformation experiments using the −2 dilution culture for each strain. Cells were naturally transformed following the protocol previously published8 with some adjustments. Briefly, at each time point (every half hour), 2 mL of cells were harvested by centrifugation at 10,000g for 1 min at 4 °C, resuspended in 2 mL of fresh CS2, and equally divided into two tubes. One or 5 µg of donor-DNA (plasmid or chromosome) was added to one of the tubes (the second tube is used as a “no DNA” control) and incubated at 37 °C for 2.5 h with agitation at 180 rpm. Ten or 100 µL (tube with DNA) or 1 mL (“no DNA control”) from each tube where finally mixed with 25 mL of melted BHI agar pre-cooled to 55 °C together with an antibiotic, and the mixture was poured into Petri dishes. After solidification, the plates were incubated at 37 °C for 48 h. At each time point, the viability was also evaluated by serial dilution on BHI agar plates. Transformation efficiencies were finally calculated by dividing the number of transformants detected in 1 mL of culture by the total number of cells in the same volume. Numbers presented in Fig. 1c represent the mean of the highest transformation efficiencies detected along growth during each experiment. The experiments have been repeated for each strain at least 5 times to provide strong statistical relevance.
Donor DNA preparation
Plasmid. The pCN34 plasmid (Kan45) was used in some of the genetic transformation experiments (Fig. 1c). pCN34 was purified from the St197 strain. Briefly, 50 mL of culture were harvested by centrifugation and the plasmid was purified using a plasmid purification kit (Macherey-Nagen).
Chromosomal DNA. strain St294 was used to provide donor chromosomal DNA (Fig. 1c, d). In St294, the pIMAY-INT14 plasmid (Cm) was inserted in the chromosome at the INT chromosomal site14. The plasmid insertion was verified by PCR while no replicating plasmid could be detected. Briefly, 100 mL of culture were centrifuged and resuspended in 5 mL of TEG (Tris 5 mM, pH8; EDTA, 10 mM; Glucose, 1%) complemented with 500 µL of Proteinase K (10 mg/mL), 2 mL of lysis buffer (NaOH, 0.2 N; SDS, 1%) and 20 g of glass beads (Stratech, #11079-105, 0,5 mm in diameter). The cells were then broken using 5 cycles of vortex (1 min each) with 1 min in ice between each cycle. To finish cell lysis, 3 mL of lysis buffer were added for 5 min at room temperature and neutralized with 6 mL of NaAc (3 M, pH 4.8). Finally, chromosomal DNA present in the supernatant was precipitated using 96% ethanol (1 ml of EtOH for 500 µL of supernatant) after 2 hours of incubation at −20 °C. After centrifugation, chromosomal DNA was washed using 300 µL of cold 70% ethanol. Precipitated chromosomal DNA was finally resuspended in 300 µL of Tris 5 mM, pH8.
RNA-sequencing
Sampling and isolation. Cultures of S. aureus were grown in CS2 medium at 37 °C and 180 rpm until the OD600 reached 2. To quench cellular metabolism / transcription and to stabilize RNAs, cells were harvested by centrifugation at 10,000g for 1 min at 4 °C and the pellets immediately frozen in liquid nitrogen before storage at −80 °C. Three independent biological replicates were collected for each of the four strains (wild type, St29; ΔcomK1, St40; ΔcomK2, St41; ΔsigH, St61). For extraction of RNA, cells were lysed using Lysing Matrix B and a FastPrep instrument (both MP Biomedicals), and RNA were isolated using the RNeasy Mini Kit (Qiagen). RNAs were treated with TURBO DNase (Ambion), purified using the RNA Cleanup protocol from the RNeasy Mini Kit (Qiagen), and stored at −80 °C. The integrity of the RNA was finally analyzed using an Agilent Bioanalyzer (Agilent Technologies).
rRNA depletion, library construction and sequencing. Removal of 23 S, 16 S, and 5 S rRNA using the RiboZero rRNA Removal Kit (Epicenter) (two times), strand-specific library construction yielding fragments of size range 100–500 bp, pooling of the 12 indexed libraries, sequencing in one flow-cell lane on a Illumina HiSeq2000 instrument with a 75 nt paired end protocol, and demultiplexing of the 12 samples of indexed reads was performed by the “Next Generation Sequencing (NGS) Core Facility” from the Institute for the Integrative Biology of the Cell (I2BC, Gif sur Yvette, France).
Read mapping and analysis of differential expression. Differential expression of all annotated features was assessed using the R statistical programming environment. Differential expression was determined between the wild-type strain samples (St29, n = 3) and each of the 9 samples (each n = 3) in which sigH, comK1, or comK2 were absent. The output from the differential expression analysis is presented in Supplementary Tables 2–5. Genes with false discovery rate (FDR)-corrected P-values < 0.01 and a ratio of differential expression superior to 2, 3, or 5 were considered significantly differentially expressed and are presented.
Data accessibility. The whole set of RNA-seq data is compiled and accessible under the GEO submission GSE224932.
S. aureus exoproteome analysis
Exoprotein isolation. S. aureus cultures (wild type, St12; ΔsigH, St45 and ΔcomK2, St 38) were grown in 10 ml CS2 in 50 mL Falcon tubes for 19.5 h. Culture supernatants were collected by centrifugation at 6000 rpm for 10 min (4 °C) to remove bacteria, followed by filtration through a 0.22 µm filter to remove cell debris. The proteins in the culture supernatants were precipitated in 20% (v/v) trichloroacetic acid (TCA) at 4 °C overnight. The precipitated proteins were sedimented by centrifugation at 13000 rpm for 45 min (4 °C) and the pellets were washed with 96% ethanol. The protein pellets were finally centrifuged at 13,000 rpm for 30 min (4 °C), the remaining ethanol was removed and the pellets were allowed to air dry.
Exoprotein profiling. Precipitated exoproteins were resuspended in 18 µl of 1× PBS and incubated for 30 min at room temperature. After addition of 20 µl of 2× Tris-Glycine SDS Novex buffer (ThermoFisher) and 1 M DTT, the samples were incubated for 10 min at 95 °C. Exoproteins were finally separated in a 4–12% Tris-Glycin gel (Invitrogen) and visualized using Coomassie staining.
Exoprotein sample preparation and LC-MS analysis. The bands containing the whole sample were cut and subjected to in-gel trypsin digestion using standard conditions including reduction and alkylation. Trypsin-generated peptides were analyzed by nanoLC–MSMS using a nanoElute liquid chromatography system (Bruker) coupled to a timsTOF Pro mass spectrometer (Bruker). Peptides were loaded on an Aurora analytical column (ION OPTIK, 25 cm × 75 m, C18, 1.6 m) and separated with a gradient of 0–35% of solvent B for 100 min. Solvent A was 0.1% formic acid and 2% acetonitrile in water and solvent B was acetonitrile with 0.1% formic acid. MS and MS/MS spectra were recorded from m/z 100 to 1700 with a mobility scan range from 0.6 to 1.4 V s/cm2. MS/MS spectra were acquired with the PASEF (Parallel Accumulation Serial Fragmentation) ion mobility-based acquisition mode using a number of PASEF MS/MS scans set as 10.
Data analysis. MS and MSMS raw data were processed and converted into mgf files with DataAnalysis software (Bruker). Protein identifications were performed using the MASCOT search engine (Matrix Science, London, UK) against S. aureus database. Database searches were performed using trypsin cleavage specificity with two possible missed cleavages. Carbamidomethylation of cysteines was set as fixed modification and oxidation of methionines as variable modification. Peptide and fragment tolerances were set at 10 ppm and 0.05 Da, respectively. Proteins were validated when identified with at least two unique peptides. Only ions with a score higher than the identity threshold and a false-positive discovery rate of less than 1% (Mascot decoy option) were considered. Mass spectrometry based-quantification was performed by label-free quantification using spectral count method. Total MS/MS spectral count values were extracted from Scaffold software (version Scaffold 4.11.1, Proteome software Inc, Portland, OR) filtered with 95% probability and 0.1% FDR for protein and peptide thresholds, respectively. For statistical analysis, missing values occurring in spectral count datasets at protein-level were imputed by a constant value fixed at 0.1. In order to take into account within-sample variation in spectral count datasets, a beta-binomial test was performed based on triplicates MS/MS analyses with P-values calculated using R package ‘ibb’ (version 13.06, 61). Proteins were filtered on a P-value < 0.05 and a fold change larger than two.
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD040550 and 10.6019/PXD040550.
Oxygen concentration measurements
Oxygen concentrations were measured using the SP-PSt3-SA23-D3-OIW oxygen sensor spots (PreSens GmbH, Regensburg, Germany). These sensor spots were attached to the inner wall of 50 mL Falcon tubes with silicone glue so that the spots would always be immerged during the experiments (i.e., below the 5 mL mark). The Falcon tubes were closed at T0 and remained closed for the entire experiment.
The sensor spots are covered with an oxygen-sensitive coating where molecular oxygen quenches the luminescence of an inert metal porphyrine complex immobilized in an oxygen-permeable matrix. This process guarantees a high temporal resolution and a measurement without drift or oxygen consumption.
The photoluminescence lifetime of the luminophore within the sensor spot was measured using a polymer optical fiber linked to an oxygen Meter (Fibox 4 trace; PreSens GmbH). Excitation light (505 nm) was supplied by a glass fiber, which also transported the emitted fluorescence signal (600 nm) back to the oxygen meter. Briefly, an oxygen measurement was realized, through the Falcon tube plastic, by simply approaching the optical fiber from the sensor spot. At each time point, the oxygen concentration was measured three times and the results provided represent the mean of these three measurements. In our experiments, the oxygen concentration was measured every 30 min.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The whole set of RNA-seq data is compiled and accessible under the GEO submission GSE224932. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD040550 and 10.6019/PXD040550. All other data are available from the corresponding author on reasonable request. Source data is available as Supplementary Data table.
References
Gordon, R. J. & Lowy, F. D. Pathogenesis of methicillin‐resistant Staphylococcus aureus infection. Clin. Infect. Dis. 46, S350–S359 (2008).
Klevens, R. M. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. J. Am. Med. Assoc. 298, 1763 (2007).
Dietz, I., Jerchel, S., Szaszák, M., Shima, K. & Rupp, J. When oxygen runs short: the microenvironment drives host–pathogen interactions. Microbes Infect. 14, 311–316 (2012).
Beenken, K. E. et al. Global gene expression in Staphylococcus aureus biofilms. J. Bacteriol. 186, 4665–4684 (2004).
Hiramatsu, K. et al. Multi-drug-resistant Staphylococcus aureus and future chemotherapy. J. Infect. Chemother. 20, 593–601 (2014).
Centers for Disease Control and Prevention. (CDC). Biggest Threats. Available at http://www.cdc.gov/drugresistance. (2013).
Lindsay, J. A. Genomic variation and evolution of Staphylococcus aureus. Int. J. Med. Microbiol. 300, 98–103 (2010).
Morikawa, K. et al. Expression of a cryptic secondary sigma factor gene unveils natural competence for DNA transformation in Staphylococcus aureus. PLoS Pathog. 8, e1003003 (2012).
Solomon, J. M. & Grossman, A. D. Who’s competent and when: regulation of natural genetic competence in bacteria. Trends Genet. 12, 150–155 (1996).
van Sinderen, D. et al. comK encodes the competence transcription factor, the key regulatory protein for competence development in Bacillus subtilis. Mol. Microbiol. 15, 455–462 (1995).
Meibom, K. L., Blokesch, M., Dolganov, N. A., Wu, C. Y. & Schoolnik, G. K. Chitin induces natural competence in Vibrio cholerae. Science 310, 1824–1827 (2005).
Lee, M. S. & Morrison, D. A. Identification of a new regulator in Streptococcus pneumoniae linking quorum sensing to competence for genetic transformation. J. Bacteriol. 181, 5004–5016 (1999).
Morikawa, K. et al. A new staphylococcal sigma factor in the conserved gene cassette: functional significance and implication for the evolutionary processes. Genes Cells 8, 699–712 (2003).
Fagerlund, A., Granum, P. E. & Havarstein, L. S. Staphylococcus aureus competence genes: mapping of the SigH, ComK1 and ComK2 regulons by transcriptome sequencing. Mol. Microbiol. 94, 557–579 (2014).
Chung, Y. S., Breidt, F. & Dubnau, D. Cell surface localization and processing of the ComG proteins, required for DNA binding during transformation of Bacillus subtilis. Mol. Microbiol. 29, 905–913 (1998).
Solomon, J. M., Magnuson, R., Srivastava, A. & Grossman, A. D. Convergent sensing pathways mediate response to two extracellular competence factors in Bacillus subtilis. Genes Dev. 9, 547–558 (1995).
Moreno-Gámez, S. et al. Quorum sensing integrates environmental cues, cell density and cell history to control bacterial competence. Nat. Commun. 8, 854 (2017).
Lo Scrudato, M. & Blokesch, M. The regulatory network of natural competence and transformation of Vibrio cholerae. PLoS Genet. 8, e1002778 (2012).
Le, K. Y. & Otto, M. Quorum-sensing regulation in staphylococci—an overview. Front. Microbiol. 6, 1174 (2015).
Maree, M. et al. Natural transformation allows transfer of SCCmec-mediated methicillin resistance in Staphylococcus aureus biofilms. Nat. Commun. 13, 2477 (2022).
Berka, R. M. et al. Microarray analysis of the Bacillus subtilis K-state: genome-wide expression changes dependent on ComK. Mol. Microbiol. 43, 1331–1345 (2002).
Ogura, M. et al. Whole-genome analysis of genes regulated by the Bacillus subtilis competence transcription factor ComK. J. Bacteriol. 184, 2344–2351 (2002).
Dagkessamanskaia, A. et al. Interconnection of competence, stress and CiaR regulons in Streptococcus pneumoniae: competence triggers stationary phase autolysis of ciaR mutant cells. Mol. Microbiol. 51, 1071–1086 (2004).
Peterson, S. N. et al. Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol. Microbiol. 51, 1051–1070 (2004).
Kato, F., Yoshizumi, S., Yamaguchi, Y. & Inouye, M. Genome-wide screening for identification of novel toxin-antitoxin systems in Staphylococcus aureus. Appl. Environ. Microbiol. 85, e00915–e00919 (2019).
Liu, Q., Yeo, W. & Bae, T. The SaeRS two‐component system of Staphylococcus aureus. Genes 7, 81 (2016).
Yarwood, J. M., McCormick, J. K. & Schlievert, P. M. Identification of a novel two-component regulatory system that acts in global regulation of virulence factors of Staphylococcus aureus. J. Bacteriol. 183, 1113–1123 (2001).
Schlag, S. et al. Characterization of the oxygen-responsive NreABC regulon of Staphylococcus aureus. J. Bacteriol. 190, 7847–7858 (2008).
Sun, F. et al. AirSR, a [2Fe-2S] cluster-containing two-component system, mediates global oxygen sensing and redox signaling in Staphylococcus aureus. J. Am. Chem. Soc. 134, 305–314 (2012).
Claverys, J.-P. P., Prudhomme, M. & Martin, B. Induction of competence regulons as a general response to stress in gram-positive bacteria. Annu. Rev. Microbiol. 60, 451–475 (2006).
Lichev, A., Angelov, A., Cucurull, I. & Liebl, W. Amino acids as nutritional factors and (p)ppGpp as an alarmone of the stringent response regulate natural transformation in Micrococcus luteus. Sci. Rep. 9, 11030 (2019).
Macfadyen, L. P. et al. Competence development by Haemophilus influenzae is regulated by the availability of nucleic acid precursors. Mol. Microbiol. 40, 700–707 (2001).
Findlay Black, H. et al. A competence-regulated toxin-antitoxin system in Haemophilus influenzae. PLoS ONE 15, e0217255 (2020).
Chan, W. T. & Espinosa, M. The Streptococcus pneumoniae pezAT toxin–antitoxin system reduces β-lactam resistance and genetic competence. Front. Microbiol. 7, 1322 (2016).
Jaskólska, M., Stutzmann, S., Stoudmann, C. & Blokesch, M. QstR-dependent regulation of natural competence and type VI secretion in Vibrio cholerae. Nucleic Acids Res. https://doi.org/10.1093/nar/gky717 (2018).
Cafini, F. et al. Methodology for the study of horizontal gene transfer in Staphylococcus aureus. J. Vis. Exp. https://doi.org/10.3791/55087-v (2017).
Cordero, M. et al. The induction of natural competence adapts staphylococcal metabolism to infection. Nat. Commun. 13, 1525 (2022).
Balasubramanian, D., Harper, L., Shopsin, B. & Torres, V. J. Staphylococcus aureus pathogenesis in diverse host environments. Pathog. Dis. https://doi.org/10.1093/femspd/ftx005 (2017).
Wilde, A. D. et al. Bacterial hypoxic responses revealed as critical determinants of the host-pathogen outcome by TnSeq Analysis of Staphylococcus aureus invasive infection. PLOS Pathog. 11, e1005341 (2015).
Vitko, N. P., Spahich, N. A. & Richardson, A. R. Glycolytic dependency of high-level nitric oxide resistance and virulence in Staphylococcus aureus. MBio 6, e00045–15 (2015).
Kreiswirth, B. N. et al. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305, 709–712 (1983).
Fey, P. D. et al. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio. 4, e00537-12 (2013).
Pourcel, C. et al. Improved multiple-locus variable-number tandem-repeat assay for Staphylococcus aureus genotyping, providing a highly informative technique together with strong phylogenetic value. J. Clin. Microbiol. 47, 3121–3128 (2009).
Monk, I. R., Shah, I. M., Xu, M., Tan, M.-W. & Foster, T. J. Transforming the untransformable: application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis. MBio 3, e00277–11 (2012).
Charpentier, E. et al. Novel cassette-based shuttle vector system for gram-positive bacteria. Appl. Environ. Microbiol. 70, 6076–6085 (2004).
Acknowledgements
We thank Kazuya Morikawa and Tarek Msadek for providing S. aureus strains and constructs and for the scientific discussions. We also want to thank the Imagerie-Gif flow cytometry and proteomics (SICaPS) facilities (Institute for the Integrative Biology of the Cell, I2BC, Gif sur Yvette, FRANCE) for their help and support. We also acknowledge the sequencing and bioinformatics expertize of the I2BC High-throughput sequencing facility, supported by France Génomique (funded by the French National Program “Investissement d’Avenir” ANR-10-INBS-09).
This work was supported by a “Young Researcher grant” from the French National Research Agency to Nicolas Mirouze (ANR-18-CE35-0004 GenTranSa) and a Chinese Scholarship Council (CSC) PhD grant awarded to Shi Yuan Feng.
Author information
Authors and Affiliations
Contributions
S.Y.F.: Conceptualization, methodology, investigation; Y.H.: Conceptualization, methodology, investigation; F.M.: Conceptualization, methodology, investigation; R.M.: Methodology, investigation; N.M.: Conceptualization, supervision, writing—review & editing, project administration and funding acquisition
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: George Inglis and Tobias Goris. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Feng, S.Y., Hauck, Y., Morgene, F. et al. The complex regulation of competence in Staphylococcus aureus under microaerobic conditions. Commun Biol 6, 512 (2023). https://doi.org/10.1038/s42003-023-04892-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-04892-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.