Abstract
Apical hooks are functional innovations only observed in angiosperms, which effectively protect the apical meristems out of damage during plant seedlings penetrating soil covers. Acetyltransferase like protein HOOKLESS1 (HLS1) in Arabidopsis thaliana is required for hook formation. However, the origin and evolution of HLS1 in plants are still not solved. Here, we traced the evolution of HLS1 and found that HLS1 originated in embryophytes. Moreover, we found that Arabidopsis HLS1 delayed plant flowering time, in addition to their well-known functions in apical hook development and newly reported roles in thermomorphogenesis. We further revealed that HLS1 interacted with transcription factor CO and repressed the expression of FT to delay flowering. Lastly, we compared the functional divergence of HLS1 among eudicot (A. thaliana), bryophytes (Physcomitrium patens and Marchantia polymorpha) and lycophyte (Selaginella moellendorffii). Although HLS1 from these bryophytes and lycophyte partially rescued the thermomorphogenesis defects in hls1-1 mutants, the apical hook defects and early flowering phenotypes could not be reversed by either P. patens, M. polymorpha or S. moellendorffii orthologs. These results illustrate that HLS1 proteins from bryophytes or lycophyte are able to modulate thermomorphogenesis phenotypes in A. thaliana likely through a conserved gene regulatory network. Our findings shed new light on the understanding of the functional diversity and origin of HLS1, which controls the most attractive innovations in angiosperms.
Similar content being viewed by others
Introduction
The process of land colonization is a hallmark event during plant evolution, which then causes the rapid radiation of angiosperms (aka. Darwin’s abominable mystery). Angiosperms indeed innovate some unique features, which facilitate their adaptations to environmental changes. For example, apical hooks in angiosperm seedlings are elegant evolutionary innovations. Newly germinated angiosperm (for instance Arabidopsis thaliana) seedlings form apical hooks to protect their shoot apical meristems during soil penetration. Once the shoot emerges from the soil, the hook opens and the cotyledons expand for photosynthesis. Dark-grown etiolated seedlings also form apical hooks.
Plants sense the depth and texture of the soil as a mechanical signal, which coordinates the biosynthesis of ethylene1. Ethylene triggers exaggerated hook formation, a feature of the so-called triple response2. Studies in the reference plant A. thaliana have uncovered the signaling paradigms underlying hook development. A forward genetics screen for mutants defective in hook formation identified the hookless1 (hls1) mutant in the early 1990s2,3. The hls1-1 mutant (in which the glutamic acid at position 346 was changed to lysine, HLS1E346K) does not display any hook formation when grown in the dark, even in the presence of ethylene, but it shows normal ethylene responses (inhibited growth) in the hypocotyl and root2. Screening for suppressors in the hls1-1 mutant background revealed that AUXIN RESPONSE FACTOR 2 (ARF2) is required for HLS1 function4.
HLS1 promotes hook formation by controlling the asymmetric distribution of auxin between the concave and convex sides of the hypocotyl, leading to differential cell growth3. In addition to functioning as a key regulator of differential cell growth, HLS1 is also recognized as a molecular hub that integrates various exogenous and endogenous cues, such as light4, auxin4, jasmonate5,6, gibberellic acid7, and salicylic acid8, to modulate hook angle.
Although HLS1 has been cloned for more than two decades, its biochemical feature is enigmatic. HLS1 encodes a protein similar to GCN5 acetyltransferase3 but it is still not clear whether HLS1 is a bona fide acetyltransferase. HLS1 is required to modulate the histone H3 acetylation (H3Ac) levels at the WRKY33 and ABA INSENSITIVE 5 (ABI5) loci, as revealed by chromatin-immunoprecipitation-qPCR (ChIP-qPCR) assays. However, recombinant HLS1 proteins purified from Escherichia coli did not have acetyltransferase activity9. This discrepancy suggests that HLS1 might act indirectly to modulate histone acetylation. A recent study argued that oligomerization of HLS1 is required for its function, and that light triggers the deoligomerization of HLS1 via a direct interaction between HLS1 and the photoreceptor phytochrome B (phyB)10. This photo-responsive deoligomerization of HLS1 inactivates HLS1 and results in hook opening. Although this study presented an updated model for illustrating HLS1 function, how the light signal is transmitted from HLS1 to its downstream targets remains a mystery.
On another side, increasing evidence show that HLS1 might be a multi-functional molecule in plants, in addition to its founding role in hook development. HLS1 directly interacts with PHYTOCHROME-INTERACTING FACTOR4 (PIF4) and co-regulates hypocotyl elongation under high ambient temperatures (i.e., thermomorphogenesis)11,12. Quadruple mutants lacking HLS1 and its three homologs display abnormal embryo patterning, dwarf architecture, and floral defects in adult plants13, suggesting that HLS plays a pivotal role during plant development.
Although HLS1 is necessary for a variety of developmental events, its origin, and evolutionary history are still not solved. We also do not know whether HLS1 functions in any other signaling pathways and do not understand when its function diverges during evolution. In this study, we tried to use phylogenomic approaches to trace the origin of HLS1 in plants and accidentally found the role of HLS1 in plant flowering time control.
Flowering at the appropriate time ensures optimal plant fitness and reproduction, which belongs to another functional innovation in angiosperms. Environmental cues, endogenous hormone signals, and plant age are dedicatedly integrated to control flowering time. FLOWERING LOCUS T (FT), the long-sought florigen protein, moves from leaf vascular tissue to the apex, where it induces flowering14,15. FT mRNA is specifically expressed in vascular tissues, and its expression is tightly controlled by multiple transcription factors, including CONSTANS (CO) and CRY2-interacting bHLH1 (CIB1)16,17,18. These transcription factors integrate environmental cues and endogenous hormone signals to precisely activate FT expression, thus ensuring that the plant flowers at the correct time. Both CO and CIB1 bind to the promoter of FT to induce its transcription. Moreover, FT expression peaks at dusk when CO protein abundance and mRNA expression are at their maximum19,20. CIB1 was the first blue light-dependent protein shown to interact with the blue light photoreceptor cryptochrome 2 (CRY2)17. CIB1 also physically interacts with CO to coordinate FT induction and flowering21.
Here, we first traced the origin of HLS1 and found that HLS1 originated in bryophytes (~480 Ma), but did not exist in chlorophytes or charophytes. Then we reported that HLS1 in A. thaliana repressed FT expression and delayed plant flowering time. Further investigations showed that HLS1 directly interacted with transcription factor CO and abrogated its transcriptional activity. Lastly, we overexpressed HLS1 orthologs from either Physcomitrium patens, Marchantia polymorpha, or Selaginella moellendorffii into hls1-1 mutants and compared their complementation phenotypes. Interestingly, HLS1 orthologs from bryophytes and lycophyte rescued the thermomorphogenesis defects in hls1-1 mutants, but could not function in apical hook development and flowering. Therefore, our results substantiated the function of HLS1 in plant flowering time control and provided an evolutionary view of the origin and functional divergence of HLS1, one of the enigmatic genes in plants.
Results
HLS1 originates in embryophytes
To trace the origin of HLS1 in plants, we used similarity search and phylogenetic analysis to identify the orthologs of A. thaliana HLS1 (AtHLS1) in Archaeplastida using both genomic and transcriptomic data (Supplementary Data 1). We found that AtHLS1 homologs with conserved functional domains and motifs existed in land plants (embryophytes), but did not occur in glaucophyte, rhodophytes, chlorophytes and charophytes (Fig. 1 and Fig. S1). Phylogenetic analyses demonstrated that AtHLS1 orthologs duplicated and resulted in multiple paralogous clades in embryophytes, probably due to the several whole genome duplication events (Fig. 1). Tree topology indicated AtHLS1 orthologs were subdivided into three major groups with strong supports (Group A, B and C) (Fig. 1). Group C included AtHLS1 orthologs from bryophytes, lycophyte and ferns, whereas Group A and B only presented in seed plants (Fig. 1). Group B included AtHLS1 orthologs from gymnosperms, while Group A contained AtHLS1 and AtHLS1 orthologs from angiosperms (Fig. 1). Extensive lineage- or clade-specific duplications occurred during the evolution of HLS1 that lead to massive paralogs of HLS1 in land plants, such as the two different groups in ferns and gymnosperms, respectively (Fig. 1), whereas only HLS1 in M. polymorpha and Ginkgo biloba presented single-copy. Our results indicated that AtHLS1 orthologs originated in embryophytes and likely duplicated in the ancestors of angiosperms, resulting in two different paralogous groups of HLS1 in flowering plants (Fig. 1). We also compared the conserved motif structures and the presence of two conserved residues (L327 and E346 in AtHLS1, as their individual point mutation identified in hls1-6 (L327W) or hls1-1 (E346K)) among these HLS1 orthologs. We found that almost all of them contained the five conserved motifs in their N-terminals and two conserved residues in C-terminals, except for one paralog of HLS1 in Gnetum montanum (Fig. 1 and Fig. S1). Taken together, our results demonstrated that AtHLS1 orthologs originated in land plants and experienced duplications and divergence in flowering plants, which may correlate with their functional divergence during plant evolution.
HLS1 delays flowering and represses FT expression
During the propagation of hls1-1 mutants in the greenhouse, we noticed that these mutants flowered earlier than the wild-type controls. To study how HLS1 might regulate flowering time, we confirmed the early-flowering phenotype in two additional, nonallelic hls1 mutants (hls1-27 and hls1-28)12. All three hls1 mutant alleles displayed earlier flowering than the wild type under our growth conditions (16 h light/8 h dark, long-day) (Fig. 2). To further check the relationship between early-flowering and hls1 mutation, we characterized flowering phenotypes in a genetic complementation line (35S:MYC-HLS1/hls1-1). This complementation line successfully rescued the apical hook defects in hls1-1 mutants (Fig. S2a), while it also reversed the early-flowering phenotypes (Fig. S2b–d). These results illustrate that HLS1 inhibits the initiation of flowering in plants.
a Images show representative flowering phenotypes of 22-day-old plants grown under LD conditions (16 h light/8 h dark). b Statistical analysis of the days from germination to flowering under LD conditions. Significant differences were determined by one-way analysis of variance (ANOVA) and post hoc Tukey’s test, with different lowercase letters indicating significant differences (data are means ± SD; n = 27, P < 0.05). c Statistical analysis of the total leaf number under LD conditions. Significant differences were determined by two-way analysis of variance (ANOVA) and post hoc Tukey’s test, with different lowercase letters indicating significant differences (data are means ± SD; n = 27, P < 0.05).
Next, we investigated whether HLS1 alters FT expression to delay flowering. FT is specifically expressed in leaf vascular tissue. Using an HLS1 promoter-driven glucuronidase (GUS) reporter system (pHLS1:GUS), we discovered that HLS1 was expressed not only in leaf mesophyll cells but also in vascular tissues (Fig. 3a). We then observed GUS activity in the pHLS1:GUS plants at different time points of day and found that pHLS1:GUS was expressed at higher levels around dusk (Fig. 3b). We also performed quantitative reverse-transcription PCR (qRT-PCR) to further confirm the HLS1 expression pattern during three consecutive long days. Consistent with the GUS staining results, HLS1 mRNA levels were elevated during the daytime, peaked at dusk, and decreased at night (Fig. 3c). These spatial and temporal expression patterns of HLS1 were coincident with the expression profiles of FT, suggesting that HLS1 might regulate FT transcription. In fact, FT expression levels were higher in the hls1-1 mutants than in the wild type, especially at dusk (Fig. 3d). Taken together, these results demonstrate that HLS1 delays flowering time and represses FT expression.
a Representative image showing GUS staining in the third leaves of pHLS1:GUS plants grown under LD for 12 days. b Representative image showing GUS staining at different Zeitgeber times in pHLS1:GUS plants grown under LD for 12 days. White bar means daytime and black bar indicates nighttime. c qRT-PCR showing HLS1 expression in 12-day-old Col-0 plants for three consecutive days (LD). White bar means daytime and black bar indicates nighttime. Significant differences were determined by one-way analysis of variance (ANOVA) and post hoc Tukey’s test, data are means ± SD; n = 3. d qRT-PCR showing FT expression levels in 12-day-old plants for three consecutive days (LD). White bar means daytime and black bar indicates nighttime. Significant differences were determined by one-way analysis of variance (ANOVA) and post hoc Tukey’s test, data are means ± SD; n = 3.
HLS1 interacts with CO
We hypothesized that HLS1 suppresses the transcriptional activators of FT to repress this gene’s expression. To uncover which factor(s) might be involved in this process, we cloned a group of flowering- or light signaling-related transcription factors, and used them as prey proteins to test protein–protein interactions (with HLS1 as a bait) individually in yeast two-hybrid assays. These assays revealed that HLS1 interacted with CO (Fig. 4a). Yeast two-hybrid assays also showed that the carboxyl end of CO (CO dNt: amino acids 133-374) interacted with HLS1 (Fig. 4a). Firefly luciferase complementation imaging (LCI) assays showed that HLS1 interacted with CO in planta (Fig. 4b). We also carried out bimolecular fluorescence complementation (BiFC) assays and found that the CO dNt fragment interacted with HLS1 but not the hls1-1 mutated form of HLS1 (HLS1E346K) (Fig. 4c). Taken together, we identified that CO is an HLS1 interacting protein and may contribute to the HLS1-regulated flowering time control.
a Yeast two-hybrid assays demonstrating that HLS1 interacts with CO. Truncated forms of CO are shown on the top. Numbers indicate the amino acid positions. b LCI assay showing that HLS interacts with CO in N. benthamiana leaves. A. tumefaciens cells harboring each construct were infiltrated into different areas of N. benthamiana leaves. After 2–4 days of infiltration, luciferin was sprayed onto the leaf surfaces and LUC activity was recorded. c BiFC assay showing HLS1-CO interactions. A. tumefaciens cells containing the indicated plasmids were co-infiltrated into N. benthamiana leaves for 2 days. YFP fluorescence was monitored under a confocal laser-scanning microscope. The YFP signal (green) channel and the nuclear marker VirD2NLS-mCherry signal (red) channel were merged with bright field images. Bars = 20 μm. nYFP, N-terminal fragment of YFP; cYFP, C-terminal fragment of YFP.
HLS1 suppresses the transcriptional activity of CO
Because the HLS1-interaction domain in CO includes its DNA-binding domain21, we assumed that HLS1 could compete with CO to repress FT expression. We first performed ChIP-qPCR and showed that HLS1 had two major binding regions in the FT promoter (region areas were adapted from22): one in a remote region (FT-C area) and the other in the same site that binds CO (FT-G area) (Fig. 5a). Then, we directly tested the regulation of FT promoter transcriptional activity. CO-enhanced luciferase (LUC) expression driven by the FT promoter (529 bp ahead of the start codon, including CO binding sites). However, simultaneously expressing HLS1 with CO dramatically repressed LUC activity (Fig. 5b), confirming that HLS1 represses the transcriptional activity of CO in vivo. To further confirm whether HLS1 represses flowering via CO, we further investigated the genetic relationship between these two genes. The co loss-of-function mutants (co-2) flowered late under long-day conditions (Fig. S3a), whereas the early-flowering phenotype of hls1-1 was largely suppressed in the hls1-1 co-2 double mutants (genotyped in Fig. S3b) (Fig. S3). Because co-2 allele is in Landsberg (Ler) background, we also crossed hls1-1 with a co mutant in Columbia background (co-9). The homozygous line (#21) of hls1-1 co-9 double mutants (genotyped in Fig. S4) displayed late flowering phenotypes compared with the hls1-1 parental lines (Fig. 5c–e), similar to the wild-type plants Col-0. These results demonstrate that the role of HLS1 in flowering time control is largely dependent on CO. Furthermore, because FT expression levels were elevated in hls1-1 (Fig. 3d), we characterized the flowering phenotypes of hls1-1 ft-10 double mutants (genotyped in Fig. S3d) and found that these mutants displayed almost identical flowering phenotypes to the ft-10 loss-of-function mutants (Fig. S3c). We then quantified the expression levels of FT in the parental lines and the hls1-1 co-9 double mutants. The high expression levels of FT in hls1-1 mutants were largely repressed by the loss of CO in the hls1-1 co-9 double mutants (Fig. 5f). These results indicated that the repression of FT expression by HLS1 mainly relied on the presence of CO transcription factor. Taken together, these results demonstrate that HLS1 interacts with CO and occupies the same binding sites in the FT promoter as CO to repress FT expression.
a ChIP-PCR assays examining the DNA-binding ability of HLS1. Cross-linked chromatin extracted from 35S:MYC-HLS1/hls1-1 plants was precipitated with anti-MYC antibody, and the eluted DNA was used to amplify different areas of the FT promoter by qPCR. Col-0 plants were used as negative controls. Values shown are means ± SD; n = 3. TSS: transcription start site; TTS: transcription terminal site. b Transient transcriptional regulation of FT promoter activity. A. tumefaciens cells harboring pFT:LUC (reporter) were co-infiltrated with different effectors into N. benthamiana leaves. LUC activity was detected at 3 days after infiltration. Representative infiltrated N. benthamiana leaf image detected under CCD camera were shown on the left side. Quantitative results (right side) showing luminescence signal counts per second (cps) in each infiltrated region. Significant differences were determined by one-way analysis of variance (ANOVA) and post hoc Tukey’s test, data are means ± SD; n = 3, *P < 0.05. c Representative image showing 24-day-old plants grown under LD conditions. d Statistical analysis of the days from germination to flowering under LD conditions. Significant differences were determined by one-way analysis of variance (ANOVA) and post hoc Tukey’s test, with different lowercase letters indicating significant differences (Data are means ± SD; n = 18, P < 0.05). e Statistical analysis of the total leaf number under LD conditions. Significant differences were determined by two-way analysis of variance (ANOVA) and post hoc Tukey’s test, with different lowercase letters indicating significant differences (Data are means ± SD; n = 18, P < 0.05). f Expression levels of FT in 7-day-old plants grown under LD conditions at ZT16. Significant differences were determined by one-way analysis of variance (ANOVA) and post hoc Tukey’s test, with different lowercase letters indicating significant differences (Data are means ± SD; n = 6, P < 0.05).
Functional divergence of HLS1 orthologs
Our phylogenomic studies have revealed the origin of HLS1 (Fig. 1), then we are wondering about the HLS1 functional divergence during evolution. We selected HLS1 orthologs from bryophytes and lycophyte as ancestral HLS1 for further investigations. In bryophytes, there are one HLS1 ortholog in M. polymorpha (liverworts) and two HLS1 orthologs in P. patens and Sphagnum fallax, respectively (mosses). There are also two HLS1 orthologs in S. moellendorffii (lycophyte). Protein sequence alignments in these orthologs and A. thaliana AtHLS1 showed that there were two highly conserved regions. One is in their amino terminus, which harbors the conserved acetyltransferase domain (Fig. 1 and Fig. S5), the other is in their carboxyl end. Interestingly, two amino acids (L327 and E346), whose mutation results in hookless phenotype in A. thaliana, were strikingly conserved among A. thaliana, M. polymorpha, P. patens and S. moellendorffii (Fig. S5). Therefore, we hypothesized that HLS1 orthologs from bryophytes and lycophyte might function in A. thaliana to a certain extent.
We initially overexpressed one HLS1 gene from M. polymorpha (MpHLS1), two HLS1 genes from P. patens (PpHLS1-1 and PpHLS1-2), and two HLS1 genes from S. moellendorffii (SmHLS1-1 and SmHLS1-2) fused with the LUC tag into hls1-1 mutants, respectively. With the help of LUC imaging, we easily identified transgenic lines expressing LUC-MpHLS1, LUC-PpHLS1-1, LUC-PpHLS1-2, LUC-SmHLS1-1, or LUC-SmHLS1-2, respectively (Fig. S6). Homozygous lines in their propagated generations were used for further studies.
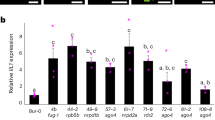
Then we examined three representative phenotypes which are controlled by HLS1. First, we checked thermomorphogenesis responses. HLS1 is required for high ambient temperature responsive hypocotyl elongation11,12. In hls1-1 mutants, their hypocotyls could not elongate under high temperature due to the defects in hypocotyl cell elongation under high temperature12. However, overexpression of each HLS1 ortholog successfully rescued the thermomorphogenesis defects in hls1-1 mutants (Fig. 6a–d). We also detected the expression levels of YUCCA8 (YUC8), which are up-regulated under high temperature in a HLS1 and PIF4 dependent manner11. Our results showed that the induction defects in hls1-1 mutants were rescued in these complementation lines (Fig. 6e). On another side, we found that no matter hls1-1 mutants or complementation lines displayed similar root elongation responses under high temperature (Fig. S7), which suggested that HLS1 is not involved in root thermomorphogenesis. It makes sense because HLS1 acts in a PIF4 dependent manner11, while PIF4 is not involved in root thermomorphogenesis according to several reports23,24. Therefore, we concluded that HLS1 from M. polymorpha, P. patens or S. moellendorffii indeed functioned well in the regulation of cell elongation during plant thermomorphogenesis (Fig. 9). Second, we looked into their flowering time. As we demonstrated, hls1-1 mutants exhibited early-flowering phenotypes. Overexpression of HLS1 orthologs from bryophytes or lycophyte in hls1-1 mutants still flowered early, which meant that these orthologs could not complement hls1-1 mutants in flowering time control (Fig. 7). Lastly, we observed their hook phenotypes. In etiolated seedlings, plants form closed cotyledons and bended apical hooks (Fig. 8a). Strong mutant alleles of hls1 (such as hls1-1) did not display any hook angles and also exhibited opened cotyledons. However, none of these heterologous overexpression lines displayed any hook angles (Fig. 8b), in contrast to the results from A. thaliana HLS1 (AtHLS1) complementation lines (Fig. S2a). Ethylene promoted exaggerated apical hook formation, we also tested whether these transgenic lines were responsive to ethylene. Our results showed that even in the presence of ethylene biosynthesis precursor 1-aminocyclopropane-1-carboxylic acid (ACC), these heterologous overexpression lines could not form any hook angles in hls1-1 mutant backgrounds (Fig. 8c). Therefore, we demonstrated that the HLS1 orthologs from bryophytes and lycophyte were not functional in the regulation of apical hook or flowering time (Fig. 9). That is to say, the function of HLS1 in the control of cell elongation (thermomorphogenesis) is ancient, but its role in hook development or flowering is obtained in eudicots.
a Representative images showing the hypocotyl phenotypes in seedlings grown under 22 °C or 28 °C. Scale bar = 1 mm. b Representative images showing the hypocotyl cells in the seedlings grown under 22 °C or 28 °C. Bars = 100 μm. c Quantification of hypocotyl length. Significant differences were determined by two-way analysis of variance (ANOVA) and post hoc Tukey’s test, with different lowercase letters indicating significant differences (Data are means ± SD; n = 60, P < 0.05). d Quantification of hypocotyl cell length. Significant differences were determined by two-way analysis of variance (ANOVA) and post hoc Tukey’s test, with different lowercase letters indicating significant differences (Data are means ± SD; n = 50, P < 0.05). e Expression levels of YUC8 in the seedlings grown under 22 °C or 28 °C. Significant differences were determined by two-way analysis of variance (ANOVA) and post hoc Tukey’s test (Data are means ± SD; n = 3, *P < 0.05, **P < 0.01).
a Images show representative flowering phenotypes of 16-day-old plants grown under LD conditions. b Statistical analysis of the days from germination to flowering under LD conditions. Significant differences were determined by one-way analysis of variance (ANOVA) and post hoc Tukey’s test, with different lowercase letters indicating significant differences (data are means ± SD; n = 18, P < 0.05). c Statistical analysis of the total leaf number under LD conditions. Significant differences were determined by two-way analysis of variance (ANOVA) and post hoc Tukey’s test, with different lowercase letters indicating significant differences (data are means ± SD; n = 18, P < 0.05).
a Illustration of the hook angle position in 4-day-old etiolated Col-0 seedling grown on MS medium. b Representative images showing hook angles in 4-day-old etiolated seedlings grown on MS medium. c Representative images showing hook angles in 4-day-old etiolated seedlings grown on MS medium supplemented with 1 μM of ACC. Scale bars = 1 mm.
According to the most up-to-date plant evolutionary relationships39 and our results, we demonstrated that the functions of HLS1 in the regulation of thermomorphogenesis (elongated seedling cartoon) were conserved from bryophytes to eudicots (shown as check marks). However, the HLS1 orthologs in lycophyte or mosses could not function in flowering time control (flower cartoon) and hook development (depicted as wrong marks). The cartoon was created with BioRender.com.
Discussion
HLS1 was among the last few genes, which had been cloned before the release of the Arabidopsis genome sequence, but its biochemical functions remain elusive even today. There are currently two models to explain HLS1 activities. The first model proposes that HLS1 directly acetylates histones. Based on protein sequence similarities, HLS1 has been recognized as a putative acetyltransferase for almost two decades, and the histone H3 acetylation (H3Ac) levels are altered in hls1 mutants. However, isolated HLS1 protein failed to directly acetylate histones in an in vitro enzymatic assay9; thus, the acetyltransferase activity of HLS1 is still under debate. In order to answer whether acetyltransferase activity is required for HLS1 function, we also generated GFP-HLS1V108A L151A/hls1-1 transgenic plants, which harbored two point mutations (V108 and L151) in the conserved acetyltransferase domain (Fig. S5 and Fig. S8). The V108 and L151 amino acids are highly conserved not only in plants but also in bacterial, yeast, and human N-acetyltransferase3. All the individual transgenic lines harboring the HLS1V108A L151A mutations could not complement the hls1-1 early-flowering phenotypes, while the normal GFP-HLS1 complementation lines successfully rescue hls1-1 mutants (Fig. S8). Although we did not directly test the acetyltransferase activity in HLS1, our results suggest that at least these two conserved amino acids in the acetyltransferase domain are required for HLS1 function.
The second model proposes that HLS1 forms oligomers in darkness. Light-activated phytochromes directly interact with HLS1 and reduce its oligomerization status to inhibit hook development10. In addition, a very recent study showed that SUMO E3 ligase SAP AND MIZ1 DOMAIN-CONTAINING LIGASE1 (SIZ1) mediates HLS1 SUMOylation and revealed six SUMOylation sites (lysine [K] 62, K81, K155, K186, K294, and K336) in HLS1 protein. Mutation of these SUMOylation sites disturbs the HLS1 oligomerization status and functions in apical hook development25. Interestingly, we mapped all these six Arabidopsis HLS1 SUMOylation sites with other orthologs and found that the K62, K294, and K336 sites are completely distinct in bryophytes or lycophyte (Fig. S5). The K81/K155 sites in S. moellendorffii are identical with A. thaliana HLS1 but not the same in M. polymorpha and P. patens. The K186 site is not changed in M. polymorpha and P. patens but is different in S. moellendorffii (Fig. S5). Since it has been demonstrated that the oligomerization status of HLS1 is correlated with its role in hook development10,25, we suspect that the un-conserved SUMOylation sites in HLS1 orthologs might explain why these HLS1 orthologs could not complement the hls1-1 hook defects.
On another side, the exact role of HLS1 in hook development is unknown. Although it is no doubt that HLS1 is required for apical hook development and asymmetric auxin distributions4, the signaling mechanisms from HLS1 to the auxin intercellular or intracellular transport are not clear. We only understand that ethylene directly induces HLS1 expression through the activation of transcription factor EIN34,13, but the downstream events from HLS1 are enigmatic.
In the present study, we tried to address these questions through a different angle. We first traced the origin of HLS1 in plants and found that HLS1 originated in embryophytes (Fig. 1). We did not identify any HLS1 orthologs in algae species, suggesting that the functions of HLS1 are likely related to the adaptation of land.
Then we revealed a novel physiological function of HLS1 and uncovered its mechanisms. We demonstrated that HLS1 interacted with CO (Fig. 4), associated with the CO binding site in the target promoter (FT) (Fig. 5a), and regulated FT transcription (Fig. 3d and Fig. 5b). This unexpected signaling mechanism of HLS1 in flowering time control is reminiscent of its role in thermomorphogenesis, which directly interacts with PIF4 and co-regulates a plenty of PIF4-target gene expressions11. HLS1 acts as positive regulator in thermomorphogenesis and negative regulator in flowering time control, respectively. Therefore, HLS1 functions as a scaffold protein that associates with multiple transcriptional regulators during different stages of plant growth and development. This unified model is reminiscent of findings for FRIGIDA (FRI), another protein involved in regulating flowering time in A. thaliana. FRI forms a supercomplex with histone acetyltransferases and histone methyltransferases that localizes to the transcriptional regulatory regions of FLOWERING LOCUS C (FLC)26. In future, an in vivo co-immunoprecipitation coupled mass-spectrometry (co-IP/MS) analysis could determine whether the HLS1 protein complex contains other proteins and reveal their identities.
Lastly, we determined the functional divergence of HLS1 from different linages. Interestingly, we found that HLS1 orthologs from M. polymorpha, P. patens, or S. moellendorffii could complement the thermomorphogenesis defects in hls1-1 mutants (Fig. 6), but could not rescue the early-flowering phenotypes and the hookless phenotypes in etiolated seedlings in hls1-1. (Figs. 7–8). These results indicate that HLS1 proteins from bryophytes or lycophyte are able to modulate cell elongation phenotypes in A. thaliana likely through a conserved gene regulatory network. In fact, it has been reported that cell elongation was also an ancestral ethylene response. In contrast to inhibition of cell elongation in A. thaliana, ethylene actually promotes cell elongation in Charophyta27. On another side, although we did not find any HLS1 orthologs in charophytes, the counterpart of EIN3 transcription factor in Charophyta (Spirogyra pratensis) can partially rescue the A. thaliana ein3 mutants (35S-SpEIN3-YFP/ein3-1) and even trigger exaggerated hook formation when overexpressed in Col-0 background (35S-SpEIN3/Col-0)27. Therefore, the role of EIN3 in charophyte is conserved with A. thaliana ortholog, but HLS1 functions more divergently during evolution. We believe that the future knock-out of HLS1 in the model plant P. patens and M. polymorpha will tell the role of HLS1 in bryophytes and shed new light on our understanding of this enigmatic protein.
We also discovered that the mutant form of HLS1 in hls1-1, which has a single amino acid substitution (HLS1E346K), could not interact with transcription factor CO (Fig. 4c). This result indicates that this amino acid (E346) is essential for the function of HLS1 in the regulation of CO activity. However, the E346 site is highly conserved in our analysis (Fig. 1 and Fig. S5) and could not explain why other HLS1 orthologs are not able to complement hls1-1 early-flowering phenotypes if E346 matters. We assumed that the protein structure or SUMOylation in HLS1 orthologs might affect their interactions with their CO counterparts in M. polymorpha, P. patens, or S. moellendorffii. It is noteworthy to test their individual HLS1-CO interactions and compare predicted protein structural differences in future.
Taken together, we reported the origin and evolutionary history of HLS1 and pointed out the functional divergence of HLS1 in different plant linages.
Materials and methods
HLS1 ortholog identification and phylogenetic analyses
To obtain orthologs of HLS1 from Archaeplastida, we performed similarity search using genomes from 32 representative plants, comprising 23 streptophytes (land plants and charophytes), 6 chlorophytes, 2 rhodophytes, and 1 glaucophyte (Supplementary Data 1). Arabidopsis AtHLS1 (At4g37580) was used as template to perform BLASTp searches against theses plant proteomes with low stringency (E value < 0.01). We also used BLASTp algorithm to search against the Phytozome v13 (https://phytozome-next.jgi.doe.gov/) and 1KP dataset (1000 plant transcriptomes, https://db.cngb.org/onekp/) to obtain putative HLS1 orthologs from glaucophytes, rhodophytes, chlorophytes, and charophytes as much as possible. We further analyzed the function domains of Arabidopsis HLS1 (At4g37580, PF00583, “N-acetyltransferase” domain) for filtering these similar sequences with hits (E value < 0.01). The filtered protein sequences were separately aligned using MAFFT (v7)28, and then trimmed by trimAL (v1.3) with -gt = 0.0329. The multiple sequence alignment was manually checked for quality and ensured the completeness of function domains. The maximum likelihood phylogenetic tree was reconstructed using IQ-TREE 230. The best-fitting model was determined by ModelFinder31, and branch supports were evaluated using the ultrafast bootstrap (UFBoot) approach and SH approximate likelihood ratio test (SH-aLRT test) with 1000 replicates32,33. The motifs were predicted by the MEME suit version 5.5.0 (https://meme-suite.org/meme/tools/meme).
Plant materials and growth conditions
The Arabidopsis hls1-1 (point mutation line), hls1-27 (SALK_136528, T-DNA inserstion line), and hls1-28 (SM_3_50, transposon insertion line) mutants used in this study were described previously3,12. The co-2 and ft-10 mutants were obtained from The European Arabidopsis Stock Centre (NASC). The co-9 mutants were described before34. The hls1-1 co-9, hls1-1 co-2, and hls1-1 ft-10 double mutants were generated by genetic crosses between hls1-1 and co-2 and between hls1-1 and ft-10, respectively. Homozygous double mutants were verified by PCR-based sequencing in areas flanking the mutated sites. Genotyping primers were listed in Supplementary Data 2. For the complementation test, 35S:MYC-HLS1/hls1-1 plants (overexpression of the MYC tagged HLS1 coding sequence driven by the 35S promoter in hls1-1 background) have been described7. The coding sequences of MpHLS1, PpHLS1-1, PpHLS1-2, and SmHLS1-1, and SmHLS1-2 were commercially synthesized (GENEWIZ) and further cloned into pEGAD-LUC vector35 to obtain LUC-fusion constructs. The mutated AtHLS1 V108A L151A fragments were generated by following the instruction manual of the Mut Express MultiS Fast Mutagenesis Kit V2 (Vazyme). The coding sequences of AtHLS1 and AtHLS1 V108A L151A were cloned into pEGAD-GFP vector35 to obtain the GFP-fusion constructs. Each construct was then individually transfected into Agrobacterium tumefaciens GV3101. A. tumefaciens strains harboring each construct were then transformed into hls1-1 plants through flowering dip method. Obtained transformants were initially screened on Basta containing MS medium (Sigma-Aldrich, #M5519). Basta-resistant lines were picked up and transferred into soil for further immuno-blot confirmation.
To observe thermomorphogenesis phenotypes, seedlings germinated on MS medium were firstly grown under 22 °C (16 h light/8 h dark) for three days and then kept at 22 °C or 28 °C for additional four days. Hypocotyls were imaged under a dissecting microscope (Nikon) and then measured with Image J software (https://imagej.nih.gov/ij/). Statistics were carried out with Prism (GraphPad Software). To examine flowering time, seeds were first placed on MS medium. After stratification for three days, the plates were incubated in a growth chamber for one week. The seedlings were transferred to soil and grown under long day (16 h light/8 h dark, 22 °C) conditions as indicated until flowering. To observe hook angles, seeds were placed on MS medium or MS supplemented with 1 μm of ACC. After stratification for 3 days, the plates were exposed with white light irradiation for three hours and then kept in complete darkness for four days. Hook angles were recorded under a dissect microscope (Nikon).
Yeast two-hybrid assay
The coding sequences of HLS1, CO, and their truncated forms were PCR amplified, cloned into the pGBKT7 or pGADT7 vector, and transformed into yeast strain AH109 using the Matchmaker Gold Yeast Two-Hybrid System according to the user manual (Clontech, #630489). Transformed yeast cells were streaked onto SD (−Leu/−His/−Ade/−Trp) medium and grown at 28 °C for 4–7 days. The white colonies represented protein–protein interactions. Primers are listed in Supplementary Data 2.
RNA extraction and qRT-PCR
Seedlings grown in the light were harvested and ground into powder for RNA extraction. Total RNA was extracted from the samples with TRIzol Reagent (Invitrogen, #15596206). Reverse transcription (Vazyme, #R223) and quantitative PCR were performed according to the manufacturer’s instructions (Vazyme, #Q111-02). Expression analysis was performed with three biological replicates. The relative expression levels were normalized against ACTIN7 as an internal control. Primers used in this study are listed in Supplementary Data 2.
GUS staining assay
12-day-old seedlings grown in the light (LD) were collected at different Zeitgeber times (ZT) and incubated in GUS staining solution at 37 °C for equal amounts of time. After staining, the seedlings were washed with 75% ethanol and observed under a stereoscopic microscope (Nikon) for imaging.
Chromatin-immunoprecipitation PCR
Two-gram samples of 10-day-old seedlings grown under LD conditions at ZT-16 were collected and cross-linked in 1% formaldehyde according to the standard ChIP protocol5. EZview Red Anti-c-Myc Affinity gel (Sigma-Aldrich, #E6654) was used to immunoprecipitate the DNA that bound to MYC-HLS1. After elution, qPCR was performed to examine interactions. The various regions of the FT promoter were previously described22. Primers used in this assay are listed in Supplementary Data 2.
Firefly luciferase complementation imaging assay (LCI)
The coding sequence of HLS1 was cloned into pCAMBIA1300-cLUC, while the coding sequence of CO was cloned into pCAMBIA1300-nLUC36. Agrobacterium tumefaciens GV3101 cells harboring the indicated constructs was infiltrated into Nicotiana benthamiana leaves using the standard protocol37. Luciferase activity was detected with a CCD camera (Tanon, #4100). In each analysis, at least three biological replications were performed with similar results.
Transient transcriptional activity assay
The pFT:LUC reporter construct was described previously38. For the effector constructs, the coding sequence of CO was cloned into pCambia1300-HA, while the coding sequence of HLS1 was cloned into pEGAD to generate GFP-HLS1. A. tumefaciens carrying the reporter or effector construct was cultured to OD600 = 0.5, and the cultures were combined for infiltration. Luciferase activity was recorded with a CCD camera (Tanon, #4100) or quantitatively measured under a luminometer (Tecan).
BiFC assays
The coding sequence of CO dNT was cloned into the pEarleyGate201-YN vector, while HLS1 or HLS1E346K was cloned into the pEarleyGate 202-YC vector, using the Gateway system (Invitrogen). The constructs were introduced into A. tumefaciens strain GV3101 and co-expressed in N. benthamiana leaves. After two days of incubation, the fluorescent signal of yellow fluorescent protein (YFP) was detected under a confocal laser-scanning microscope. The nuclear marker mCherry-VirD2NLS was simultaneously co-expressed for indicating nuclei positions in leaf pavement cells.
Statistics and reproducibility
Statistical analyses of data were performed using GraphPad Prism 7.0 software. The hypocotyl length, hypocotyl cell length, and root length were quantified by ImageJ software. Results are shown as the means ± standard deviation (SD). Statistical significance was analyzed by one-way or two-way analysis of variance and post hoc Tukey’s test, with different lowercase letters indicating significant differences (P < 0.05). Significance levels are: *P < 0.05; **P < 0.01; ***P < 0.001.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data that support the findings of this study are available from the corresponding author upon request. Source data for hypocotyl length underlying the graphs are provided in Supplementary Data 3. Accession numbers: these sequences were deposited in GenBank under the following accession numbers: MpHLS1 (ON210989), PpHLS1-1 (ON210990), PpHLS1-2 (ON210991), SmHLS1-1 (ON210992), SmHLS1-2 (ON210993).
References
Zhong, S. et al. Ethylene-orchestrated circuitry coordinates a seedling’s response to soil cover and etiolated growth. Proc. Natl. Acad. Sci. USA 111, 3913–3920 (2014).
Guzman, P. & Ecker, J. R. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2, 513–523 (1990).
Lehman, A., Black, R. & Ecker, J. R. HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell 85, 183–194 (1996).
Li, H., Johnson, P., Stepanova, A., Alonso, J. M. & Ecker, J. R. Convergence of signaling pathways in the control of differential cell growth in Arabidopsis. Dev. Cell 7, 193–204 (2004).
Zhang, X. et al. Jasmonate-activated MYC2 represses ETHYLENE INSENSITIVE3 activity to antagonize ethylene-promoted apical hook formation in Arabidopsis. Plant Cell 26, 1105–1117 (2014).
Song, S. et al. Interaction between MYC2 and ETHYLENE INSENSITIVE3 modulates antagonism between jasmonate and ethylene signaling in Arabidopsis. Plant Cell 26, 263–279 (2014).
An, F. et al. Coordinated regulation of apical hook development by gibberellins and ethylene in etiolated Arabidopsis seedlings. Cell Res. 22, 915–927 (2012).
Huang, P. et al. Salicylic acid suppresses apical hook formation via NPR1-mediated repression of EIN3 and EIL1 in Arabidopsis. Plant Cell https://doi.org/10.1105/tpc.19.00658 (2019).
Liao, C. J., Lai, Z., Lee, S., Yun, D. J. & Mengiste, T. Arabidopsis HOOKLESS1 regulates responses to pathogens and abscisic acid through interaction with MED18 and acetylation of WRKY33 and ABI5 chromatin. Plant Cell 28, 1662–1681 (2016).
Lyu, M. et al. Oligomerization and photo-deoligomerization of HOOKLESS1 controls plant differential cell growth. Dev. Cell 51, 78–88.e73 (2019).
Jin, H., Lin, J. & Zhu, Z. PIF4 and HOOKLESS1 impinge on common transcriptome and isoform regulation in thermomorphogenesis. Plant Commun 1, 100034 (2020).
Jin, H. & Zhu, Z. HOOKLESS1 is a positive regulator in Arabidopsis thermomorphogenesis. Sci. China Life. Sci. 62, 423–425 (2019).
Chang, K. N. et al. Temporal transcriptional response to ethylene gas drives growth hormone cross-regulation in Arabidopsis. eLife 2, e00675 (2013).
Corbesier, L. et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316, 1030–1033 (2007).
Lifschitz, E. et al. The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc Natl Acad Sci USA 103, 6398–6403 (2006).
Putterill, J., Robson, F., Lee, K., Simon, R. & Coupland, G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80, 847–857 (1995).
Liu, H. et al. Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 322, 1535–1539 (2008).
Kobayashi, Y., Kaya, H., Goto, K., Iwabuchi, M. & Araki, T. A pair of related genes with antagonistic roles in mediating flowering signals. Science 286, 1960–1962 (1999).
Valverde, F. et al. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 303, 1003–1006 (2004).
Song, Y. H., Smith, R. W., To, B. J., Millar, A. J. & Imaizumi, T. FKF1 conveys timing information for CONSTANS stabilization in photoperiodic flowering. Science 336, 1045–1049 (2012).
Liu, Y. et al. CIB1 and CO interact to mediate CRY2-dependent regulation of flowering. EMBO Rep. 19, e45762 (2018).
Zhai, Q. et al. Transcriptional mechanism of jasmonate receptor COI1-mediated delay of flowering time in arabidopsis. Plant Cell 27, 2814–2828 (2015).
Borniego, M. B., Costigliolo-Rojas, C. & Casal, J. J. Shoot thermosensors do not fulfil the same function in the root. New Phytol 236, 9–14 (2022).
Lee, S., Wang, W. & Huq, E. Spatial regulation of thermomorphogenesis by HY5 and PIF4 in Arabidopsis. Nat. Commun. 12, 3656 (2021).
Xiong, J. et al. Inhibition of SIZ1-mediated SUMOylation of HOOKLESS1 promotes light-induced apical hook opening in Arabidopsis. Plant Cell https://doi.org/10.1093/plcell/koad072 (2023).
Li, Z., Jiang, D. & He, Y. FRIGIDA establishes a local chromosomal environment for FLOWERING LOCUS C mRNA production. Nat. Plants 4, 836–846 (2018).
Ju, C. et al. Conservation of ethylene as a plant hormone over 450 million years of evolution. Nat. Plants 1, 14004 (2015).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Capella-Gutierrez, S., Silla-Martinez, J. M. & Gabaldon, T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973 (2009).
Minh, B. Q. et al. Corrigendum to: IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 37, 2461 (2020).
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A. & Jermiin, L. S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589 (2017).
Guindon, S. et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321 (2010).
Minh, B. Q., Nguyen, M. A. & von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 30, 1188–1195 (2013).
Cheng, Z. et al. Nup96 and HOS1 are mutually stabilized and gate CONSTANS protein level, conferring long-day photoperiodic flowering regulation in arabidopsis. Plant Cell 32, 374–391 (2020).
Cutler, S. R., Ehrhardt, D. W., Griffitts, J. S. & Somerville, C. R. Random GFP::cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc. Natl. Acad. Sci. USA 97, 3718–3723 (2000).
Chen, H. et al. Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol. 146, 368–376 (2008).
Sun, K., Zheng, Y. & Zhu, Z. Luciferase complementation imaging assay in nicotiana benthamiana leaves for transiently determining protein-protein interaction dynamics. J. Vis. Exp. https://doi.org/10.3791/56641 (2017).
Zhang, R., Yang, C., Jiang, Y. & Li, L. A PIF7-CONSTANS-centered molecular regulatory network underlying shade-accelerated flowering. Mol. Plant. 12, 1587–1597 (2019).
Zhang, Z. et al. Origin and evolution of green plants in the light of key evolutionary events. J. Integr. Plant Biol. 64, 516–535 (2022).
Acknowledgements
We thank Dr. Lin Li for the pFT:LUC plasmid, Dr. Chentao Lin for pEGAD-LUC vector, Dr. Yongfu Fu for the BiFC vectors, and Dr. Shiyong Song for co-9 mutants. This study was supported by the National Natural Science Foundation of China (31970256), the Natural Science Foundation of Jiangsu Province (BK20201371), the Key Laboratory of Molecular Design for Plant Cell Factory of Guangdong Higher Education Institutes (2019KSYS006), the QingLan Project of Jiangsu Province and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Contributions
Z.Zhu. designed the research. Q.W. and R.W. generated transgenic plants and performed most of the experiments. N.L. and H.J. characterized flowering phenotypes and initiated yeast two-hybrid assays. J.S. and Z.Zhang. performed evolutionary studies. B.Z. commented on phylogeny and revised manuscript. All authors analyzed data. Z.Zhu. wrote the manuscript with inputs from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Javier Moreno and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: José Estevez and Luke R. Grinham. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Q., Sun, J., Wang, R. et al. The origin, evolution and functional divergence of HOOKLESS1 in plants. Commun Biol 6, 460 (2023). https://doi.org/10.1038/s42003-023-04849-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-04849-4
This article is cited by
-
A nonsynonymous mutation in BhLS, encoding an acyl-CoA N-acyltransferase leads to fruit and seed size variation in wax gourd (Benincasa hispida)
Theoretical and Applied Genetics (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.