Abstract
Alanyl-tRNA synthetase (AlaRS) retains a conserved prototype structure throughout its biology, consisting of catalytic, tRNA-recognition, editing, and C-Ala domains. The catalytic and tRNA-recognition domains catalyze aminoacylation, the editing domain hydrolyzes mischarged tRNAAla, and C-Ala―the major tRNA-binding module―targets the elbow of the L-shaped tRNAAla. Interestingly, a mini-AlaRS lacking the editing and C-Ala domains is recovered from the Tupanvirus of the amoeba Acanthamoeba castellanii. Here we show that Tupanvirus AlaRS (TuAlaRS) is phylogenetically related to its host’s AlaRS. Despite lacking the conserved amino acid residues responsible for recognition of the identity element of tRNAAla (G3:U70), TuAlaRS still specifically recognized G3:U70-containing tRNAAla. In addition, despite lacking C-Ala, TuAlaRS robustly binds and charges microAla (an RNA substrate corresponding to the acceptor stem of tRNAAla) as well as tRNAAla, indicating that TuAlaRS exclusively targets the acceptor stem. Moreover, this mini-AlaRS could functionally substitute for yeast AlaRS in vivo. This study suggests that TuAlaRS has developed a new tRNA-binding mode to compensate for the loss of C-Ala.
Similar content being viewed by others
Introduction
An aminoacyl-tRNA synthetase (aaRS) attaches a specific amino acid to one of its cognate tRNAs to form an aminoacyl-tRNA. This charged tRNA is then delivered to the ribosome for translation of the genetic code1. Therefore, aminoacyl-tRNA plays a key role in DNA translation―the expression of genes to make proteins. Many eukaryotic aaRSs recruit N- or C-terminal appended domains to expand their nontranslational functions during evolution. Hence, even aaRSs of the same amino acid specificity often possess distinct structural organizations between prokaryotes and eukaryotes. However, AlaRS has never recruited a new domain during evolution2. Its prototype structure consists of the catalytic, tRNA-recognition, editing, and C-terminal (C-Ala) domains3. AlaRS exists as a homodimer (or tetramer) in prokaryotes4,5 and a monomer in eukaryotes6. For instance, the hyperthermophilic archaeon Archaeoglobus fulgidus AlaRS (AfAlaRS) is a homodimer of 906 amino acid residues and genetically divided into two parts. Residues 1-739 include the catalytic, tRNA-recognition, and editing domains, while residues 736-906 comprise the C-Ala domain7. The N-terminal two domains are often referred to as the aminoacylation domain, which is responsible for alanylation of tRNAAla, and the editing domain is responsible for removal of the mischarged amino acids from tRNAAla. Defects in editing activity leads to misacylation, mistranslation, and cytotoxicity in yeast8 and neural degeneration in mice9. In contrast to the other three domains, C-Ala highly diverges among species. Prokaryotic C-Ala consists of a helical and a globular subdomain4 and constitutes the major tRNA-binding module10. The helical subdomain mediates dimer formation, whereas the globular subdomain targets the elbow of the L-shaped tRNAAla11. Despite eukaryotic C-Ala possessing a similar architecture, its helical subdomain consists of three α-helices instead of two. Human C-Ala binds DNA robustly but not tRNAAla, whereas C. elegans C-Ala binds both DNA and tRNAAla robustly6,11,12.
Identity elements of tRNA may be single nucleotides, base pairs, or post-transcriptional modifications, and often reside in the acceptor stem and anticodon loop. AlaRS establishes tRNAAla identity via a unique G3:U70 wobble base pair in the acceptor stem. G3:U70 exists in all tRNAsAla, ranging from E. coli to human cytoplasm, but is missing from all non-tRNAsAla13,14,15. Recognition of this identity element is mediated by two conserved amino acid residues, Asn (N) and Asp (D) (as N359 and D450 in A. fulgidus and as N303 and D400 in E. coli), in the tRNA-recognition domain. These two amino acid residues form specific hydrogen bonds with the G:U base pair, thereby establishing its identity as the alanine acceptor11. The amide nitrogen of the Asn side chain contacts O4 of U70 (from major-grove side), while the carboxyl side chain and the backbone carbonyl of Asp make hydrogen bonds with the 2-amino group of G3 (from minor-grove side)15,16.
Tupanvirus belongs to the family of giant viruses Mimiviridae. Its genome contains 1400–1500 kb of double-strand linear DNA17. Based on the size and sequence of its genome, Tupanvirus can be divided into two strains: soda lake and deep ocean. Both strains were first identified to replicate in Acanthamoeba castellanii and Vermamoeba vermiformis18, and later in Acanthamoeba polyphaga, Acanthamoeba griffin, Dyctistelium discoideum, and Willartia magna19. Interestingly, Tupanvirus possesses a complete set of translation-related genes, including 20 aaRSs and tRNA isoacceptors associated with all amino acids, tRNA and mRNA maturation enzymes, translation initiation, elongation, and releasing factors. Paradoxically, however, Tupanvirus does not share the same codon preference with its natural host A. castellanii. Instead, the Tupanvirus genome’s codons match its own tRNA isoacceptors18, suggesting that Tupanvirus tRNA isoacceptors may be responsible for translation of its own genome.
Among the 20 Tupanvirus aaRSs, AlaRS was of particular interest, as it contains only the aminoacylation domain. Moreover, it lacks the conserved N and D residues in its tRNA-recognition domain. This piqued our curiosity as to whether and how this mini-AlaRS can function normally. Our results showed that Tupanvirus AlaRS (TuAlaRS) can bind and charge microAla (an RNA substrate corresponding to the acceptor stem of tRNAAla) as well as tRNAAla specifically and robustly, an unexpected feature for a C-Ala-defective AlaRS. Conceivably, this mini-AlaRS has evolved a new tRNA-binding mode to counterbalance the loss of its C-Ala.
Results
TuAlaRS harbors only the aminoacylation domain
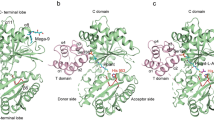
Unlike its A. fulgidus homologue, TuAlaRS possesses only the catalytic and tRNA-recognition domains (Fig. 1a). Motifs 1, 2, and 3 are still conserved in its catalytic domain. However, the conserved G3:U70-recognition amino acid residues N and D are missing from its tRNA-recognition domain. Instead, P321 and T416 are located at the corresponding positions in sequence alignment (Fig. 1b). To get a deeper insight, a structural model of TuAlaRS (with only 461 amino acid residues) was built using AlphaFold2 20 and compared to the aminoacylation domain of A. fulgidus AlaRS (AfAlaRS-N484) obtained from AfAlaRS/tRNAAla complex (PDB ID 3WQY). Both structural models were visualized and further modified using EzMol21. TuAlaRS folds into a three-dimensional structure closely resembling that of AfAlaRS-N484 (Fig. 1c). P321 and T416 of TuAlaRS are located at almost the same positions as the conserved N and D residues of AfAlaRS-N484. Nevertheless, there are still some notable differences between these two structures, in particular at their C-termini. The C-terminal region of AfAlaRS-N484 (aa 456–484) folds into two short α-helices, while the corresponding region of TuAlaRS (aa 432–461) folds into a long α-helix.
a Schematic representation of TuAlaRS and AfAlaRS. The relative positions of the four domains are marked. b Alignment of the sequences flanking the G3:U70-recognition amino acid residues N and D (highlighted in red) in the tRNA-recognition domain. c Structural modeling of TuAlaRS in comparison with AfAlaRS-N484. The catalytic and tRNA-recognition domains were colored in light-blue and purple, respectively. The C-terminal helix of the tRNA-recognition domain was highlighted in pink.
Four Tupanvirus tRNAAla isoacceptors have been recovered, each of which carries a G3:U70 base pair (Supplementary Fig. 1). One of them has a D-loop larger than normal, while the rest of them carry a normal-sized D-loop. The sequences in the TψC-loop are conserved, while those in the D-loop are relatively diverged. Nevertheless, the bases that are important for the elbow formation (such as G18G19 in the D-loop and ψ55C56 in the TψC-loop) are still conserved, suggesting that they are likely to fold into the L-shaped structure22.
TuAlaRS charges tRNAAla and microAla efficiently and specifically
As TuAlaRS lacks the major tRNA-binding module (C-Ala), we wondered whether this enzyme can charge tRNAAla efficiently. To this end, we purified the His6-tagged TuAlaRS protein from an E. coli transformant and carried out an aminoacylation assay using in vitro-transcribed E. coli tRNAAla (EctRNAAla) as the substrate. To our surprise, this mini-AlaRS was almost as efficient as EcAlaRS in charging EctRNAAla (with ~2-fold difference in charging activity) (Fig. 2a, b). In addition, despite lacking the conserved N and D residues in its tRNA-recognition domain, TuAlaRS was still specific to G3:U70 and failed to charge EctRNAAla with G3:C70 or A3:U70 to a detectable level (Fig. 2a).
The aminoacylation activities of (a) TuAlaRS and (b) EcAlaRS toward WT and mutant EctRNAAla transcripts were determined by testing their ability to incorporate 3H-alanine into the transcripts. The aminoacylation activities of (c) TuAlaRS and (d) EcAlaRS toward WT and mutant Ec-microAla transcripts were determined under similar conditions. The enzyme concentration used in the reactions was 100 nM. Error bars are standard deviation from triplicates.
We next tested whether TuAlaRS can efficiently charge a tRNAAla mimic without the elbow. Pursuant to this objective, an E. coli microhelixAla (Ec-microAla, which corresponds to the acceptor stem of EctRNAAla) was used as the substrate (Supplementary Fig. 1, TuAlaRS charged this microhelix to a higher level than did EcAlaRS when the same concentration of enzymes was used (Fig. 2c, d). As a matter of fact, TuAlaRS charged EctRNAAla and Ec-microAla to a similar level (Fig. 2a, c). Not surprisingly, mutation of G3:U70 to G3:C70 or A3:U70 in Ec-microAla dramatically impaired its aminoacylation by TuAlaRS (Fig. 2c). To determine whether Tupanvirus tRNAAla (TutRNAAla) isoacceptors are good substrates for TuAlaRS and EcAlaRS, we arbitrarily chose one of the TutRNAAla isoacceptors (second from the left in Supplementary Fig. 1A) as the substrate for aminoacylation. As shown in Supplementary Fig. 2, both enzymes could charge TutRNAAla, albeit to a level slightly lower than that of EctRNAAla (2–4-fold). In addition, we also checked whether TuAlaRS can efficiently charge its host (A. castellanii) tRNAnAla (Supplementary Fig. 1). The results showed that TuAlaRS charges A. castellanii tRNAnAla as well as EctRNAAla (Supplementary Fig. 3).
TuAlaRS binds both tRNAAla and microAla robustly
TuAlaRS’s ability to charge both EctRNAAla and Ec-microAla efficiently implies that this mini-AlaRS can bind both ligands robustly. To take a closer look, we carried out an electrophoretic mobility shift assay (EMSA) using EctRNAAla and Ec-microAla as the ligands. As shown in Fig. 3a, TuAlaRS bound EctRNAAla robustly (with a Kd value of 0.3 µM). Mutation of G3:U70 to G3:C70 or A3:U70 reduced its binding affinity 2–3-fold (Fig. 3a). EcAlaRS could also bind EctRNAAla robustly (with a Kd value of 1.0 µM; Fig. 3b). However, unlike the scenario of TuAlaRS, mutation at G3:U70 had little effect on its binding by EcAlaRS (~1.3-fold reduction in binding affinity) (Fig. 3b). Thus, mutation at G3:U70 had a stronger impact on binding by TuAlaRS than by EcAlaRS. A similar scenario was observed in mutation of the 3′-CCA end, in which deletion of the 3′-CCA end or substituting the 3′-CCA end with UUG reduced its binding by TuAlaRS 3–4-fold (Fig. 3a), but hardly affected its binding by EcAlaRS (Fig. 3b). The fact that deletion and substitution of the 3′-CCA end showed a comparable effect on TuAlaRS’s binding implies that the 3′-CCA end is important for recognition by TuAlaRS.
The binding affinities of (a) TuAlaRS and (b) EcAlaRS for WT and mutant EctRNAAla transcripts were determined by an EMSA with protein concentrations ranging from 32 to 0.0625 μM. The binding affinities of (c) TuAlaRS and (d) EcAlaRS for WT and mutant Ec-microAla transcripts were determined under similar conditions. Error bars are standard deviation from triplicates.
We next checked whether TuAlaRS can bind Ec-microAla robustly. Consistent with an earlier report23, EcAlaRS bound Ec-microAla poorly (with a Kd value of >32 µM) (Fig. 3d). However, it was interesting to find that TuAlaRS binds Ec-microAla robustly (with a Kd value of 0.3 µM) (Fig. 3c). As a result, TuAlaRS bound to EctRNAAla and Ec-microAla equally well (Fig. 3a, c), indicating that this mini-AlaRS mainly targets the acceptor stem instead of the elbow. Mutation of G3:U70 to G3:C70 or A3:U70 reduced its binding affinity distinctly, but not drastically (~3-fold increase in Kd; Fig. 3c). In contrast, an EcAlaRS fragment (N461) possessing the N-terminal 461 amino acid residues bound EctRNAAla and Ec-microAla poorly (with Kd values of >32 µM) (Supplementary Fig. 4), emphasizing the indispensable role of C-Ala in tRNAAla binding by EcAlaRS. Hence, the major binding affinity of EcAlaRS for tRNAAla arises from C-Ala targeting the elbow10, whereas the major binding affinity of TuAlaRS for tRNAAla arises from the mini-synthetase targeting the acceptor stem (Fig. 3). This might also explain why mutations in the acceptor stem have a stronger effect on binding by TuAaRS.
TuAlaRS fails to charge C. elegans mitochondrial microAla
As reported earlier, an extra G:U base pair, G1:U72, in the acceptor stem of C. elegans mitochondrial tRNAAla blocks its aminoacylation by E. coli, S. cerevisiae, and C. elegans cytoplasmic AlaRSs efficiently12,24. We wondered whether this extra G:U base pair has a similar effect on TuAlaRS. To this end, we carried out an aminoacylation assay using C. elegans mitochondrial microAla (Ce-microAla) (Supplementary Fig. 1) as the substrate. As shown in Supplementary Fig. 5A, TuAlaRS charged Ec-microAla efficiently but not Ce-microAla, suggesting that G1:U72 blocks TuAlaRS’s aminoacylation of Ce-microAla. We next checked whether this anti-determinant blocks its binding by the mini-AlaRS. Contrary to our anticipation, TuAlaRS bound Ce-microAla with affinity similar to that for Ec-microAla (with Kd values of 0.3–0.4 μM) (Supplementary Fig. 5B), suggesting that this anti-determinant affects catalysis instead of binding. Perhaps G1:U72 interferes with the positioning of the 3′-CCA end of the acceptor stem in the active-site pocket.
Kinetic parameters for aminoacylation of tRNAAla and microAla by TuAlaRS
To obtain the kinetic parameters (KM and kcat) for TuAlaRS’s aminoacylation of EctRNAAla and Ec-microAla, we performed a kinetic assay using EcAlaRS as a control. As shown in Table 1, TuAlaRS charged EctRNAAla and Ec-microAla with similar efficiency (<2-fold difference in kcat/KM), whereas EcAlaRS highly preferred EctRNAAla over Ec-microAla (~68-fold difference in kcat/KM). In addition, EcAlaRS and TuAlaRS charged EctRNAAla with similar efficiency (~2-fold difference in kcat/KM), but TuAlaRS charged Ec-microAla with a much higher efficiency than did EcAlaRS (~26-fold difference in kcat/KM). Moreover, mutation of G3:U70 to G3:C70 or A3:U70 in EctRNAAla or Ec-microAla drastically reduced its aminoacylation efficiency (~100-fold reduction in kcat/KM) by TuAlaRS, with a major impact on kcat, a scenario reminiscent of the EcAlaRS-catalyzed tRNAAla aminoacylation15. Thus, discrimination of G3:U70 by TuAlaRS mainly occurs on the kcat level rather than on the KM level.
P321 and T416 are dispensable for aminoacylation
As shown in Fig. 1, the highly conserved N and D are absent from the tRNA-recognition domain of TuAlaRS. Instead, P321 and T416 are located at the corresponding positions in sequence alignment (Fig. 1b). In addition, structural modeling showed that the P and T residues are located at almost the same positions as the N and D residues of AfAlaRS-N484 (Fig. 1c). To check whether these two amino acid residues still play a similar role in recognition of G3:U70, we mutated P and T to A individually and assayed their aminoacylation activities. As expected, mutation at P or T had little effect on the enzyme’s tRNA charging activity and specificity. Similar to the WT enzyme, P321A and T416A could efficiently charge G3:U70-containing Ec-microAla, but not G3:C70 or A3:U70-containing Ec-microAla (Supplementary Fig. 6). Thus, P321 and T416 appear to be dispensable for G3:U70 recognition.
TuAlaRS can functionally rescue a yeast ALA1 knockout (KO) strain
To investigate whether TuAlaRS can function in vivo as an AlaRS enzyme, we cloned the gene encoding TuAlaRS into a yeast shuttle vector (with a TEF1 promoter) and transformed the resultant construct into a yeast ALA1 (which encodes AlaRS) KO strain to test whether this mini-AlaRS can substitute for yeast AlaRS and rescue the growth defect of the KO strain. As shown in Fig. 4a, the KO strain could not grow in the absence of a functional AlaRS on 5-FOA. Remarkably, TuAlaRS was able to restore the growth phenotype of the KO strain on 5-FOA, albeit not as efficiently as yeast AlaRS. This result suggests that TuAlaRS can charge yeast tRNAnAla to a level sufficient for growth and that a lack of the editing domain in this mini-AlaRS does not cause cytotoxicity. Western blotting further showed that TuAlaRS is expressed to a level distinctly lower than that of yeast AlaRS under the conditions used (Fig. 4b). Thus, TuAlaRS can be a functional enzyme in vivo. In contrast, while EcAlaRS effectively rescued the growth defect of the KO strain, N461, an EcAlaRS fragment comparable to TuAlaRS, failed to do so. We wondered whether TuAlaRS shows toxicity under conditions with extra serine or glycine. To this end, we carried out complementation assays on 5-FOA with additional serine. The results showed that addition of extra serine (up to 5 mM) does not cause any toxic phenotype in the yeast knockout strain carrying TuAlaRS (Supplementary Fig. 7), suggesting that this enzyme has tolerable or low mischarging activity.
a Complementation assay. TuAlaRS’s rescue activity was determined by transforming the test plasmid into a yeast ALA1 KO strain and plating the resultant transformants on 5-FOA. The symbols “+” and “−” denote positive and negative complementation, respectively. b Western blotting. The protein expression levels of these constructs were determined by Western blotting using an anti-His6-tagged antibody as the probe. Constructs used in (a, b) are numbered for clarity.
Phylogenetic relationship of TuAlaRS with other AlaRSs
To trace the evolutionary origin of TuAlaRS and its relationships with other AlaRSs, we aligned the protein sequences of representative AlaRSs recovered from all three domains of life and built a phylogenetic tree25,26. As shown in Fig. 5, except for the mitochondrial AlaRSs of A. castellanii and D. discoideum, which were clustered into the bacterial group, all cytoplasmic and mitochondrial isoforms of eukaryotic AlaRSs tested were clustered into the same group, suggesting that these isoforms descended from common predecessors through gene duplication (Fig. 5). In addition, this main eukaryotic group showed a higher affinity for the bacterial than for the archaeal group, suggesting a bacterial origin for the eukaryotic AlaRSs. As for TuAlaRS, it was closely associated with A. castellanii and D. discoideum cytoplasmic AlaRSs and belonged to the main eukaryotic group. As A. castellanii and D. discoideum are its natural hosts, this finding implies that TuAlaRS was horizontally transferred from its host genome and the gene encoding this enzyme was reduced afterwards.
The evolutionary history was inferred by using the Maximum Likelihood method and Le_Gascuel_2008 model. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the JTT model. A discrete Gamma distribution was used to model evolutionary rate differences among sites. This analysis involved 26 amino acid sequences. Evolutionary analyses were conducted in MEGA11.
Discussion
TuAlaRS displays a G3:U70 specificity
AlaRS is the only aaRS that retains a prototype structure throughout its biology. However, Nanoarchaeum equitans harbors a split AlaRS gene that encodes an α and a β subunit27. The α-subunit, comprising an intact catalytic domain and part of the tRNA-recognition domain, charges tRNAAla with a low activity and in a non-G3:U70-specific manner. The β-subunit comprises the rest of the tRNA-recognition domain along with the editing and C-Ala domains. Addition of the β subunit into the α subunit-catalyzed aminoacylation reaction enhances its activity drastically and confers a G3:U70 specificity. Unlike N. equitans AlaRS, TuAlaRS contains an intact catalytic domain and an intact tRNA-recognition domain but lacks the editing and C-Ala domains completely (Fig. 1a). This mini-AlaRS charges G3:U70-containing tRNAAla and microAla efficiently and specifically (Fig. 2 and Table 1).
Paradoxically, TuAlaRS lacks the conserved N and D residues in its tRNA-recognition domain; instead P and T residues are located at the corresponding positions (Fig. 1). However, mutation at either amino acid residue had little effect on the enzyme’s tRNA charging activity or specificity (Supplementary Fig. 6). Clearly, TuAlaRS uses a recognition mode distinct from E. coli (which selects G3:U70 through a direct positive recognition) or eukarya/archaea (which repels a non-G3:U70 base pair) AlaRS15. Perhaps, these two amino acids play a role in the enzyme’s structural stability or flexibility. To facilitate the acceptor stem binding, the enzyme might select the universal identity element through a new site/mode. It is worth mentioning that C. elegans mitochondrial AlaRS also lacks the conserved N/D, but the enzyme still exhibits a G3:U70 specificity12,24. Human mitochondrial AlaRS, on the other hand, presents a completely different scenario, in which the conserved N/D residues are replaced by M/G and its cognate tRNA lacks G3:U70 (Fig. 1). As it turns out, this enzyme discriminates human mitochondrial tRNAAla mainly through recognition of its variable loop28. In addition, Drosophila melanogaster mitochondrial AlaRS recognizes a shifted G:U base pair ― G2:U7129.
In the case of tyrosyl-tRNA synthetase (TyrRS), an insertion peptide that splits the active site-containing domain determines the species-specific acylation. Substrate specificity can be switched by transplanting part of the insertion from human TyrRS into E. coli TyrRS and, reciprocally, by transplanting the counterpart from the E. coli enzyme into the human enzyme30. Unfortunately, there is no insertion peptide found in the active site-containing domain of AlaRS. Instead, an anti-determinant, G1:U72, is found in the acceptor stem of the C. elegans mitochondrial tRNAAla. E. coli and Tupanvirus AlaRSs fail to charge this tRNA because of the presence of G1:U72. Charging is effectively blocked by the 4-ketooxygen of U72 in the major grove31. However, adaptations make C. elegans mitochondrial AlaRS insensitive to the presence of the 4-ketooxygen. Extensive alanine-scanning mutagenesis of motif 2 of EcAlaRS provided little evidence for important contacts between tRNAAla and this segment of the protein32, which is largely consistent with the observation made in the cocrystal structure of A. fulgidus AlaRS:tRNAAla16. Perhaps, it is the flexibility of the motif 2 loop, instead of the amino acid residues therein, that enables the C. elegans mitochondrial enzyme to accommodate the 4-ketooxygen.
Despite lacking C-Ala, TuAlaRS binds tRNAAla robustly
In E. coli, C-Ala constitutes the major tRNA-binding module, targeting the elbow of the L-shaped tRNAAla10. As a result, EcAlaRS charges EctRNAAla efficiently but not Ec-microAla (by a ~60-fold difference)23 (also repeated and shown in Table 1). Deletion of C-Ala from EcAlaRS reduces its binding affinity for tRNAAla drastically (up to 13-fold)10. A similar scenario was observed for C. elegans cytoplasmic AlaRS12. Despite the fact that human cytoplasmic AlaRS can charge tRNAAla efficiently in the absence of its C-Ala6, it is unclear whether this enzyme can also efficiently charge microAla. In addition, a truncated form of Aquifex aeolicus AlaRS that contains only the aminoacylation domain (N453) can efficiently charge a G3:U70-containing minihelix (an RNA substrate corresponding to the acceptor stem plus the TψC arm)33, suggesting that its C-Ala is dispensable for tRNA binding. We demonstrated herein that TuAlaRS can bind and charge microAla and tRNAAla with a similarly high efficiency (Figs. 2 and 3 and Table 1). Clearly, TuAlaRS exclusively targets the acceptor stem of tRNAAla. However, it should be emphasized that G3:U70 in the acceptor stem still represents the major identity element for TuAlaRS’s recognition of tRNAAla (Fig. 2). Mutation at this base pair primarily affects kcat (catalysis) instead of KM (apparent affinity) for aminoacylation (Table 1), a scenario reminiscent of EcAlaRS34. To the best of our knowledge, TuAlaRS is the only naturally occurring aaRS that contains only the aminoacylation domain and can function normally.
According to the A. fulgidus AlaRS:tRNAAla complex, the amino acid residues 193GGG195 act as the route separator between reactive and non-reactive CCA-ends16. Mutation of any of these three Gly residues causes mis-aminoacylation of G3:C70-containing tRNAAla 34. Interestingly, the second and third Gly residues are conserved in TuAlaRS and other AlaRSs, implying that the remaining Gly residues play a similar role as the route separator. Moreover, unlike E. coli AlaRS, which binds the CCA-containing and CCA-deletion (or substitution) tRNAsAla with a similar affinity, TuAlaRS distinctly prefers the CCA-containing tRNAAla (Fig. 3a, b), lending further support to the hypothesis that TuAlaRS has gained additional specific contacts with the acceptor stem through the CCA-end.
TuAlaRS may have lost its editing and C-Ala domains later during evolution
Our phylogenetic analysis suggested that TuAlaRS is closely related to its hosts’ cytoplasmic AlaRSs (Fig. 5), consistent with the hypothesis that Tupanvirus acquired most of its genes through horizontal transfer from its host genomes18,35. However, gene acquisition in giant viruses such as Tupanvirus is often accompanied by gene loss or reduction in response to environmental changes during evolution. Thus, TuAlaRS’s smaller size may have resulted from gene reduction during evolution. Examples of gene reduction were also found in TyrRS of Acanthamoeba polyphaga mimivirus and many proteins of Chlorella virus36,37. Regardless of the detailed interpretation, our study suggests that TuAlaRS has developed a new binding mode to counterbalance the loss of C-Ala. Instead of targeting the elbow, this mini-AlaRS targets the acceptor stem (Fig. 3). In a sense, TuAlaRS exemplifies an ancient-like aaRS, which possesses only the aminoacylation domain and identifies its cognate tRNA through recognition of an operational RNA code (a specific sequence or structure) in the acceptor stem38.
Methods
Construction of plasmids
For expression in E. coli, the gene encoding TuAlaRS (GenBank: MF405918.2; protein ID: QKU33644.1) was codon optimized for E. coli and synthesized by Omics Biotechnology (Taiwan). The synthesized gene was cloned into the NdeI/XhoI sites of pET21b (an E. coli expression vector) and the resultant plasmid was transformed into an E. coli strain, BL21-CodonPlus(DE3). The resultant transformants were induced with 1 mM isopropyl β-D-1-thiogalactopyranoside for 4 h at 30 °C. The His6-tagged protein was purified to homogeneity through Ni-NTA affinity chromatography followed the protocols provided by the manufacture of the Ni-NTA resin (Qiagen, Hilden, Germany)39. For expression in yeast, the gene encoding TuAlaRS was codon optimized for S. cerevisiae and cloned in pTEF1 (a yeast shuttle vector with a strong TEF1 promoter).
Electrophoretic mobility shift assay (EMSA)
Protein-RNA binding affinities were determined by an EMSA, as described earlier10. The RNA substrate was 32P-radiolabeled at the 5′-end40. For each complex formation, ~10,000 cpm of RNA (diluted with cold RNA to 0.1 μM final concentration) was incubated for 10 min on ice with variable concentrations of AlaRS in a total volume of 20 μl containing 20 mM HEPES, pH 7.5, 20 mM KCl, 5 mM MgCl2, and 2 mM DTT. Glycerol was added to a final concentration of 5% in each sample prior to loading on a native 5% 29:1 polyacrylamide gel. The gel was run at 100 V for 30–45 min at 4 °C in 0.5× TBE and then dried. The dried gel was exposed to X-ray film at −80 °C with an intensifying screen. The signal intensity was compared through ImageJ to measure the remaining free 32P-RNA.
Complementation assay on FOA
Construction of a yeast haploid ALA1 KO strain (MATα, ala1::kanMX4, his3Δ1 leu2Δ0 lyS3Δ0 ura3Δ0) was described earlier41. To examine whether a heterologous AlaRS gene can functionally substitute for the cytoplasmic activity of yeast ALA1 (which encodes both the cytoplasmic and mitochondrial forms of AlaRS), a test plasmid carrying the target gene and a LEU2 marker was transformed into the KO strain, and the resultant transformant was tested for its ability to grow on 5-FOA (1 mg/ml) plates. Because 5-FOA can be converted to a toxic compound in the presence of URA3, the transformant must evict the maintenance plasmid (carrying a WT ALA1 gene and a URA3 marker) on 5-FOA to survive. Thus, the transformant cannot grow on 5-FOA unless the test plasmid encodes a functional cytoplasmic AlaRS.
In vitro transcription of tRNAAla and microAla
In vitro transcription of tRNA followed a standard protocol. We synthesized and cloned a DNA fragment containing a T7 promoter followed by a sequence encoding tRNAAla into the SmaI site of pUC18. We enriched the transcription DNA template by PCR amplification of the cloned fragment. We performed in vitro transcription at 37 °C for 3 h with 0.3 μM T7 RNA polymerase in a buffer containing 20 mM Tris-HCl (pH 8.0), 150 mM NaCl, 20 mM MgCl2, 5 mM DTT, 1 mM spermidine, and 2 mM of each NTP. We purified the tRNA transcript using an 8 M urea-15% polyacrylamide gel. Following purification, ethanol precipitation, and vacuum drying, we dissolved the tRNA pellet in 1× TE buffer (20 mM Tris-HCl [pH 8.0] and 1 mM EDTA), refolded the tRNA by heating it to 80 °C for 3 min, and gradually cooled it to 55 °C before adding 2 mM of MgCl2. We collected the refolded tRNA when the temperature reached ~25 °C and stored it at -80 °C. Preparation of microAla followed a similar protocol.
Aminoacylation assay
We performed in vitro aminoacylation assays at 37 °C in a buffer containing 50 mM HEPES (pH 7.5), 50 mM KCl, 15 mM MgCl2, 5 mM dithiothreitol, 10 mM ATP, 0.1 mg/ml bovine serum albumin, 5 μM tRNAAla (or microAla), 20 μM alanine (including 1.34 μM 3H-alanine; PerkinElmer, Waltham, MA, USA), and 100 nM AlaRS. At various times, we quenched the reactions by spotting 10 μl aliquots of the reaction mixture onto Whatman filter pads (Maidstone, Kent, UK). The filter pads had been presoaked in a solution containing 5% trichloroacetic acid and 2 mM alanine. We washed the filter pads three times for 15 min each in ice-cold 5% trichloroacetic acid and dried the pads with a heat lamp before liquid scintillation counting. We obtained aminoacylation data from three independent experiments and averaged them.
Kinetic parameters for aminoacylation of tRNAAla and microAla by AlaRS were determined by fitting the data points directly to the Michaelis-Menten equation. Initial rates of aminoacylation were determined at 37 °C with tRNAAla concentration ranging from 1 to 10 µM, and microAla concentrations ranging from 1 to 10 µM (for TuAlaRS) or 10–400 µM (for EcAlaRS). AlaRS concentrations ranged from 200 to 4000 nM. Active protein concentrations were determined by active site titration in the reaction cocktail containing [γ-32P]ATP, 10 mM MgCl2, 144 mM Tris-Cl (pH 7.8), 1 mM cognate amino acid, and yeast inorganic pyrophosphate (2 units/ml). The reaction was then incubated at 25 °C42.
Statistics and reproducibility
The data were obtained from three independent experiments and averaged.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Code availability
There is no custom code or mathematic algorithm used in this study.
References
Woese, C. R., Olsen, G. J., Ibba, M. & Söll, D. Aminoacyl-tRNA synthetases, the genetic code, and the evolutionary process. Microbiol. Mol. Biol. Rev. 64, 202–236 (2000).
Guo, M., Yang, X. L. & Schimmel, P. New functions of aminoacyl-tRNA synthetases beyond translation. Nat. Rev. Mol. Cell Biol. 11, 668–674 (2010).
Jasin, M., Regan, L. & Schimmel, P. Modular arrangement of functional domains along the sequence of an aminoacyl tRNA synthetase. Nature 306, 441–447 (1983).
Naganuma, M., Sekine, S., Fukunaga, R. & Yokoyama, S. Unique protein architecture of alanyl-tRNA synthetase for aminoacylation, editing, and dimerization. Proc. Natl Acad. Sci. USA 106, 8489–8494 (2009).
Putney, S. D., Sauer, R. T. & Schimmel, P. R. Purification and properties of alanine tRNA synthetase from Escherichia coli A tetramer of identical subunits. J. Biol. Chem. 256, 198–204 (1981).
Sun, L., Song, Y., Blocquel, D., Yang, X. L. & Schimmel, P. Two crystal structures reveal design for repurposing the C-Ala domain of human AlaRS. Proc. Natl Acad. Sci. USA 113, 14300–14305 (2016).
Fukunaga, R. & Yokoyama, S. Crystallization and preliminary X-ray crystallographic study of alanyl-tRNA synthetase from the archaeon Archaeoglobus fulgidus. Acta Crystallogr. Sect. F: Struct. Biol. Crystalliz. Commun. 63, 224–228 (2007).
Zhang, H., Wu, J., Lyu, Z. & Ling, J. Impact of alanyl-tRNA synthetase editing deficiency in yeast. Nucleic Acids Res. 49, 9953–9964 (2021).
Lee, J. W. et al. Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature 443, 50–55 (2006).
Guo, M. et al. The C-Ala domain brings together editing and aminoacylation functions on one tRNA. Science 325, 744–747 (2009).
Naganuma, M. et al. The selective tRNA aminoacylation mechanism based on a single G*U pair. Nature 510, 507–511 (2014).
Antika, T. R. et al. Gain of C-Ala enables AlaRS to target the L-shaped tRNAAla. Nucleic Acids Res. 50, 2190–2200 (2022).
Musier-Forsyth, K. et al. Specificity for aminoacylation of an RNA helix: an unpaired, exocyclic amino group in the minor groove. Science 253, 784–786 (1991).
Hou, Y. M. & Schimmel, P. A simple structural feature is a major determinant of the identity of a transfer RNA. Nature 333, 140–145 (1988).
Chong, Y. E. et al. Distinct ways of G:U recognition by conserved tRNA binding motifs. Proc. Natl Acad. Sci. USA 115, 7527–7532 (2018).
Naganuma, M. et al. The selective tRNA aminoacylation mechanism based on a single G•U pair. Nature 510, 507–511 (2014).
Claverie, J. M. & Abergel, C. Mimiviridae: An Expanding Family of Highly Diverse Large dsDNA Viruses Infecting a Wide Phylogenetic Range of Aquatic Eukaryotes. Viruses 10, 506 (2018).
Abrahao, J. et al. Tailed giant Tupanvirus possesses the most complete translational apparatus of the known virosphere. Nat. Commun. 9, 749 (2018).
Oliveira, G., La Scola, B. & Abrahao, J. Giant virus vs amoeba: fight for supremacy. Virol. J. 16, 126 (2019).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Reynolds, C. R., Islam, S. A. & Sternberg, M. J. E. EzMol: A Web Server Wizard for the Rapid Visualization and Image Production of Protein and Nucleic Acid Structures. J. Mol. Biol. 430, 2244–2248 (2018).
Zhang, J. & Ferré-D’Amaré, A. R. The tRNA Elbow in Structure, Recognition and Evolution. Life (Basel) 6, 3 (2016).
Francklyn, C. & Schimmel, P. Aminoacylation of RNA minihelices with alanine. Nature 337, 478–481 (1989).
Chihade, J. W., Hayashibara, K., Shiba, K. & Schimmel, P. Strong selective pressure to use G:U to mark an RNA acceptor stem for alanine. Biochemistry 37, 9193–9202 (1998).
Le, S. Q. & Gascuel, O. An improved general amino acid replacement matrix. Mol. Biol. Evol. 25, 1307–1320 (2008).
Tamura, K., Stecher, G. & Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 38, 3022–3027 (2021).
Arutaki, M. et al. G:U-Independent RNA Minihelix Aminoacylation by Nanoarchaeum equitans Alanyl-tRNA Synthetase: An Insight into the Evolution of Aminoacyl-tRNA Synthetases. J. Mol. Evol. 88, 501–509 (2020).
Kuhle, B., Chihade, J. & Schimmel, P. Relaxed sequence constraints favor mutational freedom in idiosyncratic metazoan mitochondrial tRNAs. Nat. Commun. 11, 969 (2020).
Lovato, M. A., Chihade, J. W. & Schimmel, P. Translocation within the acceptor helix of a major tRNA identity determinant. Embo J. 20, 4846–4853 (2001).
Wakasugi, K., Quinn, C. L., Tao, N. & Schimmel, P. Genetic code in evolution: switching species-specific aminoacylation with a peptide transplant. EMBO J. 17, 297–305 (1998).
Beuning, P. J., Gulotta, M. & Musier-Forsyth, K. Atomic Group “Mutagenesis” Reveals Major Groove Fine Interactions of a tRNA Synthetase with an RNA Helix. J. Am. Chem. Soc. 119, 8397–8402 (1997).
Davis, M. W., Buechter, D. D. & Schimmel, P. R. Functional dissection of a predicted class-defining motif in a class II tRNA synthetase of unknown structure. Biochemistry 33, 9904–9911 (1994).
Swairjo, M. A. et al. Alanyl-tRNA synthetase crystal structure and design for acceptor-stem recognition. Mol. Cell 13, 829–841 (2004).
Miller, W. T., Hou, Y. M. & Schimmel, P. Mutant aminoacyl-tRNA synthetase that compensates for a mutation in the major identity determinant of its tRNA. Biochemistry 30, 2635–2641 (1991).
Brandes, N. & Linial, M. Giant Viruses-Big Surprises. Viruses 11, 404 (2019).
Abergel, C., Rudinger-Thirion, J., Giege, R. & Claverie, J. M. Virus-encoded aminoacyl-tRNA synthetases: structural and functional characterization of mimivirus TyrRS and MetRS. J. Virol. 81, 12406–12417 (2007).
Yamada, T., Onimatsu, H. & Van Etten, J. L. Chlorella viruses. Adv. Virus Res. 66, 293–336 (2006).
Schimmel, P., Giegé, R., Moras, D. & Yokoyama, S. An operational RNA code for amino acids and possible relationship to genetic code. Proc. Natl Acad. Sci. USA 90, 8763–8768 (1993).
Chang, C. P. et al. Promoting the formation of an active synthetase/tRNA complex by a nonspecific tRNA-binding domain. J. Biol. Chem. 283, 30699–30706 (2008).
Ryder, S. P., Recht, M. I. & Williamson, J. R. Quantitative analysis of protein-RNA interactions by gel mobility shift. Methods Mol. Biol. 488, 99–115 (2008).
Tang, H. L. et al. Translation of a yeast mitochondrial tRNA synthetase initiated at redundant non-AUG codons. J. Biol. Chem. 279, 49656–49663 (2004).
Fersht, A. R. et al. Active site titration and aminoacyl adenylate binding stoichiometry of aminoacyl-tRNA synthetases. Biochemistry 14, 1–4 (1975).
Acknowledgements
This work was supported by a grant (MOST 110-2311-B-008-001) from the Ministry of Science and Technology (Taipei, Taiwan).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by T.R.A., D.J.C., Y-K.T., Y-H.Y, C-D.H., and C-C.W. The first draft of the paper was written by T.R.A., D.J.C., and C-C.W. and all authors commented on previous versions of the paper. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Jonathan Perreault and Manuel Breuer. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Antika, T.R., Chrestella, D.J., Tseng, YK. et al. A naturally occurring mini-alanyl-tRNA synthetase. Commun Biol 6, 314 (2023). https://doi.org/10.1038/s42003-023-04699-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-04699-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.