Abstract
SETD2 is a tumor suppressor that is frequently inactivated in several cancer types. The mechanisms through which SETD2 inactivation promotes cancer are unclear, and whether targetable vulnerabilities exist in these tumors is unknown. Here we identify heightened mTORC1-associated gene expression programs and functionally higher levels of oxidative metabolism and protein synthesis as prominent consequences of Setd2 inactivation in KRAS-driven mouse models of lung adenocarcinoma. Blocking oxidative respiration and mTORC1 signaling abrogates the high rates of tumor cell proliferation and tumor growth specifically in SETD2-deficient tumors. Our data nominate SETD2 deficiency as a functional marker of sensitivity to clinically actionable therapeutics targeting oxidative respiration and mTORC1 signaling.
Similar content being viewed by others
Introduction
Inactivation of SETD2 (SET domain containing 2, histone lysine methyltransferase) is a prevalent feature of many cancer types including ~7% of lung adenocarcinomas1,2,3. SETD2 has the unique catalytic activity for histone H3 lysine 36 trimethylation (H3K36me3) which it deposits along gene bodies during transcriptional elongation4. However, additional non-histone substrates have been identified which link SETD2 action with diverse roles in chromosome segregation (α-tubulin), interferon signaling (STAT1), or regulation of other chromatin modifying enzymes (EZH2)5,6,7. Loss of H3K36me3 due to SETD2 inactivation is implicated in the impairment of DNA repair, accurate splice site usage, transcriptional control, and DNA and RNA methylation8,9,10,11,12. These numerous characterized functions obscure how SETD2 mediates lung tumor suppression and whether inactivation of SETD2 presents therapeutic vulnerabilities that can be targeted to limit cancer growth.
To study Setd2 inactivation in vivo, we previously used the well-characterized, Cre-inducible KrasLSL-G12D/+ (K) and KrasLSL-G12D/+; p53flox/flox (KP) mouse models of lung adenocarcinoma. Combined with a lentiviral vector that expresses Cre recombinase, along with the essential CRISPR components, Cas9 and an sgRNA to Setd2, we and others have shown that inactivation of Setd2 in KrasG12D-driven lung adenocarcinoma promotes cellular proliferation very early after tumor initiation; an effect that is widespread amongst all developing tumors13,14. Moreover, Setd2 quantitatively ranks at the apex of all major tumor suppressors for its ability to suppress KRAS-driven cell proliferation13,15. However, unlike other tumor suppressors such as Trp53 or Rb1, whose inactivation promotes cell state changes that drive malignant progression, loss of differentiation, and metastasis, inactivation of Setd2 seems only to fuel cellular proliferation in these models14,16,17. As such, we sought to interrogate the consequences of Setd2 inactivation in KRAS-driven lung adenocarcinoma in order to better understand how SETD2 deficiency drives early and widespread tumor growth and identify intrinsic therapeutic vulnerabilities that may exist.
Results
SETD2 deficiency promotes OXPHOS and protein synthesis gene expression programs
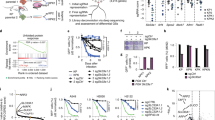
To gain mechanistic insights into how SETD2 constrains cancer growth, we performed a comprehensive analysis of RNA-sequencing data across multiple human cancer types expressing low or high levels of SETD22. Using Gene Set Enrichment Analysis (GSEA), we identified an enrichment of ribosomal- and mitochondrial-associated gene sets that negatively correlated with SETD2 expression. This association was strongly apparent in multiple tumor types that have a high frequency of SETD2 mutations, in addition to lung adenocarcinoma (Fig. 1A and Supplementary Fig. 1A–D)18. The negative correlation of ribosomal- and mitochondrial-associated gene sets was unique to SETD2 expression, as these genes sets were not associated with expression levels of other major tumor suppressors in lung adenocarcinoma such as TP53 or RB1, which instead correlated with DNA replication-associated gene sets (Supplementary Fig. 1E–G). These results suggest that ribosomal- and mitochondrial-biosynthetic pathways are activated in SETD2-deficient tumors and may be responsible for driving tumor cell proliferation. To extend this analysis, we analyzed gene expression data from KrasLSL-G12D/+ (K) tumors initiated with a non-targeting control lentiviral CRISPR vector (K-Ctrl) or an sgRNA targeting Setd2 (K-Setd2KO), as well as more advanced tumors isolated from KrasLSL-G12D/+; p53flox/flox;YFPflox/flox (KPY-Ctrl or KPY-Setd2KO) mice. In each case (K and KPY), control tumors and Setd2KO tumors were assessed via histological analysis to select stage matched specimens for comparison. K-Setd2KO tumors, which were low grade adenomas, and KPY-Setd2KO tumors, which were higher grade adenocarcinomas, had vastly different gene expression profiles than their K-Ctrl and KPY-Ctrl counterparts and a strong enrichment of ribosomal- and mitochondrial-associated gene sets. (Fig. 1B, C, Supplementary Fig. 1H, I). Collectively these results suggest that ribosomal- and mitochondrial-biosynthetic pathways are activated in SETD2-deficient tumors and may be responsible for driving increased tumor cell proliferation.
a Gene set enrichment analysis (GSEA) network plot of the 20 most enriched gene sets negatively-correlated with SETD2 expression in human lung adenocarcinomas. The size of each node corresponds to the normalized enrichment score (NES) and the width of the connecting lines indicates the number of overlapping genes between gene sets. Gene sets are categorized as mitochondrial, ribosomal or other according to the genes represented. b GSEA network plot of the top 20 enriched gene sets in KPY-Setd2KO lung adenocarcinomas. The size of each node corresponds to the normalized enrichment score (NES) and the width of the connecting lines indicates the number of overlapping genes between gene sets. Gene sets are categorized as mitochondrial, ribosomal or other according to the genes represented. c Representative mitochondrial (GO Electron Transport Chain) and ribosomal (GO Cytoplasmic Translation) GO biological process ontology gene sets that are enriched in K-Setd2KO (left) and KPY-Setd2KO (right) tumors. d Depiction of mitochondrial electron transport chain genes that show enrichment in the molecular signatures database (mSigDB) Mootha VOXPHOS gene set in K-Setd2KO tumors53. Genes are grouped according to the relevant ETC complex. Genes that are part of the core enrichment of the gene set are marked in gray, genes that are not enriched are marked in white, and mitochondrial encoded genes are outlined in red. e Depiction of ribosomal 40 S and 60 S genes that show enrichment in the mSigDB GO Ribosome Biogenesis gene set in K-Setd2KO tumors. Genes that are enriched in this gene set are marked in gray while genes that are not enriched are marked in white.
SETD2-deficient tumors have altered mitochondrial morphology and function
The major driver of enrichment for mitochondrial-associated gene sets in SETD2-deficient tumors was the significant up-regulation of genes encoding mitochondrial electron transport chain (ETC) proteins in complexes I, III, IV and V (Fig. 1D). To determine whether physical changes occur in the mitochondria when Setd2 is inactivated, we imaged KP-Ctrl and KP-Setd2KO tumors by transmission electron microscopy. Although the overall frequency of mitochondria observed per field of view was similar between genotypes (Supplementary Fig. 2A, B), the mitochondria in KP-Setd2KO tumors were significantly different than those found in KP-Ctrl tumors by several parameters. Grossly, the mitochondria of KP-Setd2KO tumors were significantly smaller and had a more electron dense matrix than controls (Fig. 2A–C Supplementary Fig. 2A). Morphologically, KP-Setd2KO mitochondria also had significantly more cristae overall, and on average each cristae was significantly more swollen (Fig. 2A, D, E)18,19. While some of these morphological changes can be associated with mitochondrial dysfunction and cell death, the higher electron density in the mitochondria matrix and a greater overall number of cristae in the mitochondria of KP-Setd2KO tumors may suggest greater oxidative function20.
a Transmission electron micrograph of mitochondria from a KP-Ctrl tumor (left) or KP-Setd2KO tumor (right). White arrows denote swollen mitochondrial cristae. Scale bars = 250 nm. b Quantification of the area of individual mitochondria measured from electron micrographs. Data indicate the mean ± standard deviation. Data points represent individual mitochondria (KP-Ctrl: n = 42 mitochondria, n = 3 mice, KP-Setd2KO: n = 54 mitochondria, n = 3 mice). Significance determined by unpaired Student’s t-test. c Quantification of the matrix electron density of individual mitochondria measured from electron micrographs. Data indicate the mean ± standard deviation. Data points represent individual mitochondria (KP-Ctrl: n = 16 mitochondria, n = 2 mice, KP-Setd2KO: n = 28 mitochondria, n = 1 mice). Significance determined by unpaired Student’s t-test. d Quantification of the number of cristae per mitochondria normalized to mitochondrial area. Data indicate the mean ± standard deviation. Data points represent individual mitochondria (KP-Ctrl: n = 37 mitochondria, n = 3 mice, KP-Setd2KO: n = 34 mitochondria, n = 3 mice). Significance determined by unpaired Student’s t-test. e Quantification of the width of individual mitochondrial cristae measured from electron micrographs. Data indicate the mean ± standard deviation. Data points represent individual cristae (KP-Ctrl: n = 179 cristae, n = 42 mitochondria, n = 3 mice, KP-Setd2KO: n = 177 cristae, n = 50 mitochondria, n = 3 mice). Significance determined by unpaired Student’s t-test. f Quantification of the total mitochondrial mass by median fluorescence intensity of MitoTracker Deep Red FM within tumor cells isolated from KPY mice. Data represent the mean ± standard deviation. Data points represent individual tumors (KPY-Ctrl: n = 9 tumors, n = 2 mice, KPY-Setd2KO: n = 6 tumors, n = 2 mice). Significance determined by unpaired Student’s t-test. Histogram shows representative flow data from KPY-Ctrl and KPY-Setd2KO tumors with unstained control. g Quantification of the mitochondrial membrane potential by median fluorescence intensity of MitoProbe DiIC1(5) within tumor cells isolated from KPY mice. Data is normalized to the mitochondrial mass of each sample. Data indicate the mean ± standard deviation. Data points represent individual tumors (KPY-Ctrl: n = 13 tumors, n = 2 mice, KPY-Setd2KO: n = 11 tumors, n = 2 mice). Significance determined by unpaired Student’s t-test. Histogram shows representative flow data from KPY-Ctrl and KPY-Setd2KO tumors with unstained control. h Seahorse XF cell mitochondrial stress test assay performed in H2009 shSetd2 and shCtrl cells. Relative oxygen consumption rate was normalized to total protein abundance. Each symbol in OCR profile plots represents the mean of at least n = 6 technical replicates of three reading cycles. OCR profile plots for (i) basal respiration (j) ATP production, and (k) maximal respiration. Each symbol represents one technical replicate per cell line. Data indicate the mean ± standard deviation. Significance determined by unpaired Student’s t-test.
To investigate mitochondrial properties in neoplastic cells directly ex vivo, we crossed a Rosa26LSL-YFP Cre-reporter allele into the KP model, generating KPY mice (Supplementary Fig. 2C)21. Consistent with the EM data, KPY-Setd2KO tumor cells had decreased staining for MitoTracker Deep Red indicative of decreased mitochondrial mass (Fig. 2F). Though KPY-Setd2KO tumor cells did not differ from KPY-Ctrl tumor cells with respect to mitochondrial superoxide production, the mitochondrial oxidative potential and the total ETC activity was significantly higher in KPY-Setd2KO tumor cells (Fig. 2G, Supplementary Fig. 2D, E).
To determine if the observed mitochondrial changes in SETD2-deficient tumors result in increased mitochondrial function and consequential ATP production, we generated cell lines expressing two distinct shRNAs targeting Setd2 in the H2009 human lung adenocarcinoma cell line that harbors oncogenic KRAS and p53 mutations22. As expected, SETD2 shRNA-expressing cell lines had decreased SETD2 and H3K36me3 compared to control cell lines, confirming a knockdown of SETD2 (Supplementary Fig. 3A–D). To profile mitochondrial function we performed a cell mitochondrial stress test assay (Seahorse XF) on these cell lines. In agreement with our in vivo data, SETD2-deficient human cell lines displayed increased oxygen consumption rates and ATP production (Fig. 2H–K). These data demonstrate that SETD2-deficiency promotes increased mitochondrial metabolism and are consistent with the gene expression programs that are enriched in human and mouse tumors with low SETD2 expression.
mTORC1 signaling and protein synthesis are heightened in SETD2-deficient tumors
An additional feature of SETD2-deficient tumors was the significant enrichment of genes associated with protein synthesis and ribosome biogenesis (Fig. 1B, C, E)23. To assess the impact of SETD2 deficiency on protein synthesis, we pulse-labeled K-Ctrl and K-Setd2KO mice with the tRNA mimetic O-propargyl-puromycin (OP-Puro). OP-Puro is incorporated into actively translated proteins to allow for quantification of protein synthesis by flow cytometry or fluorescent microscopy and offers a method to quantify the rate of protein synthesis that is compatible with small sample sizes24,25,26. Combined staining for H3K36me3 and OP-Puro incorporation demonstrated a marked enhancement of overall protein synthesis in K-Setd2KO tumors (Fig. 3A, B). The incorporation of the Rosa26LSL-YFP allele in our KPY model afforded an orthogonal approach to study differences in protein synthesis rates by measuring the brightness of cells expressing the YFP reporter protein. While KPY-Setd2KO tumor cells were only slightly larger (3.8% higher FSC-A) and mRNA expression from the Rosa26LSL-YFP allele was similar to KPY-Ctrl tumor cells, KPY-Setd2KO tumor cells had an 18.5% increase in mean YFP fluorescence. This indicates a positive effect on YFP protein synthesis without affecting YFP mRNA production. (Supplementary Fig. 4A, B). These data suggest that SETD2 normally constrains the rate of protein synthesis in addition to the degree of OXPHOS. These functions, which would be expected to limit cellular proliferation, are consistent with the proliferation-driving effects of Setd2 inactivation in KRAS-driven lung cancer.
a Representative images of o-propargyl-puromycin (OP-Puro) incorporation (red), H3K36me3 (green), and DAPI-stained nuclei (white) in K-Ctrl (left) and K-Setd2KO(right) tumors. Scale bars = 100 μm, insets are magnified 5x. b Quantification of OP-Puro mean fluorescence intensity in K-Ctrl and K-Setd2KO tumors. Data represent the mean ± standard deviation. Data points represent individual tumors (K-Ctrl: n = 18 tumors, n = 3 mice, K-Setd2KO: n = 17 tumors, n = 3 mice). Significance determined by unpaired Student’s t-test. c Representative images of phosphorylated 4E-BP1(T37/46) staining (red) in K-Ctrl (left) and K-Setd2KO(right) tumors. Nuclei are counterstained with DAPI (white). Scale bars = 100 μm, insets are magnified 5x. d Quantification of p-4E-BP1(T37/46) mean fluorescence intensity in K-Ctrl and K-Setd2KO tumors. Data represent the mean ± standard deviation. Data points represent individual tumors (K-Ctrl: n = 24 tumors, n = 3 mice, K-Setd2KO: n = 29 tumors, n = 3 mice). Significance determined by unpaired Student’s t-test. e Representative images of co-immunofluorescence of mTOR (green) and LAMP2 (red) indicating localization of mTOR at the lysosome (yellow) in K-Ctrl (left) and K-Setd2KO(right) tumors. Nuclei are counterstained with DAPI (blue). Scale bars = 10 μm, inset is magnified 2X. f Quantification of the percentage of colocalization between mTOR and LAMP2 in K- Ctrl and K-Setd2KO tumors. Data points represent individual tumors (K-Ctrl: n = 8 tumors, n = 3 mice, K-Setd2KO: n = 9 tumors, n = 3 mice). Significance determined by unpaired Student’s t-test.
Tightly linked with both protein synthesis and OXPHOS is the master nutrient sensing complex mTORC127,28,29. To determine whether SETD2 deficiency is associated with increased mTORC1 activity, we first evaluated human lung adenocarcinomas using reverse phase protein array (RPPA) data from the Cancer Proteome Atlas30,31. An established marker of mTORC1 activity is the sequential phosphorylation of the translational repressor 4E-BP1, first at Thr37/Thr46 to prime subsequent phosphorylation at Thr70 and Thr6532,33. SETD2-deficient human tumors had significantly increased phosphorylation of 4E-BP1 at Thr70, while total 4E-BP1 levels were unchanged (Supplementary Fig. 4C). Additionally, there was a significant negative correlation between SETD2 mRNA expression and phosphorylated 4E-BP1(T70) (Supplementary Fig. 4D). By profiling human lung adenocarcinoma genomic data we also found that tumors with low SETD2 expression were significantly enriched for an mTORC1 signaling-related gene set (Supplementary Fig. 4E)2. Consistent with these analyses of human datasets, we identified significantly increased mTORC1-dependent 4E-BP1(T37/46) phosphorylation in K-Setd2KO tumors compared to K-Ctrl tumors (Fig. 3C, D)34. Further, SETD2-deficient tumors had significantly higher levels of mTORC1 localized at the lysosome where it is known to actively signal, further indicating that SETD2 deficiency promotes mTORC1 activity (Fig. 3E, F).
Therapeutic targeting of OXPHOS and mTORC1 counteracts SETD2-deficient tumor growth
Our discovery that mTORC1 signaling, protein synthesis, and mitochondrial OXPHOS are increased in SETD2-deficient tumors suggested that these processes may drive cell proliferation and thus offer therapeutic susceptibilities for SETD2-mutant cancers. To assess this possibility, we treated mice bearing established K-Ctrl and K-Setd2KO tumors daily for 4 weeks with either the mTORC1 inhibitor rapamycin, the mitochondrial complex I inhibitor IACS-10759, or the anti-diabetic biguanide phenformin which inhibits both complexes (Fig. 4A)35,36,37. All three therapeutics had little effect on K-Ctrl tumors. However, each treatment significantly suppressed the increased tumor growth of K-Setd2KO tumors (Fig. 4B, C). SETD2-deficient tumors were particularly sensitive to rapamycin treatment, which had a similar suppressive effect on tumor growth as phenformin which inhibits both mitochondrial complex I and mTORC1 signaling (Fig. 4B, C). Inhibition of mTORC1 and mitochondrial complex I activity resulted in significantly reduced cell proliferation, marked by decreased phospho-H3 presence, demonstrating that the proliferative impact of SETD2 inactivation is driven, at least in part, through these pathways (Fig. 4D, Supplementary Fig. 5A). Further, inhibition of mTORC1 and mitochondrial complex I, alone or in combination, did not result in significant levels of cell death indicating that cell death is not the cause of the decreased tumor growth observed (Supplementary Fig. 5B). Phenformin is a highly potent inhibitor of both mitochondrial complex I and mTORC1. This potency has led to significant toxicity and its clinical replacement for the treatment of type II diabetes with the related biguanide metformin, which is used widely and is epidemiologically associated with suppressing cancer incidence38,39,40. Therefore, we treated K-Ctrl and K-Setd2KO mice bearing established tumors with metformin for an extended period of 12 weeks, mimicking long-term metformin treatment. While metformin treatment had no impact on K-Ctrl tumor growth, metformin-treated K-Setd2KO tumors were significantly smaller and less proliferative than vehicle treated K-Setd2KO tumors (Fig. 4E–G).
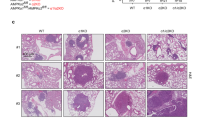
a Schematic of experiment whereby K-Ctrl or Setd2KO tumors were initiated using LentiCRISPRv2Cre. 16 weeks after tumor initiation (black line) mice were randomly assigned to vehicle or drug treatment regimen for 4 weeks (red line), at which point mice were sacrificed for assessment. For metformin treatment, mice were given the drug for 12 weeks prior to sacrifice. b Quantification of mean tumor areas in vehicle-, rapamycin-, IACS-10759-, or phenformin-treated K-Ctrl or K-Setd2KO mice. Individual tumor sizes were normalized to the mean area of vehicle-treated control mice. Box and whisker plots indicate the median, lower quartile, upper quartile, maximum and minimum data points. Data points represent individual tumors (K-Ctrl: n = 109 vehicle, n = 216 rapamycin, n = 155 IACS-10759, n = 144 phenformin; K-Setd2KO: n = 503 vehicle, n = 142 rapamycin, n = 99 IACS-10759, n = 138 phenformin). Significance determined by unpaired Student’s t-test. c Representative scans of tumor-bearing lobes from K-Ctrl or K-Setd2KO mice treated with vehicle, rapamycin, IACS-10759 or phenformin. d Quantification of cell proliferation by the percentage of p-H3 positive cells in K-Ctrl or K-Setd2KO tumors treated with vehicle, rapamycin, IACS-10759 or phenformin. Data indicate the mean ± standard deviation. Data points represent individual tumors (K-Ctrl: n = 14 vehicle, n = 14 rapamycin, n = 13 IACS-10759, n = 13 phenformin; K-Setd2KO: n = 20 vehicle, n = 14 rapamycin, n = 14 IACS-10759, n = 14 phenformin). Significance determined by unpaired Student’s t-test. e Quantification of mean tumor areas in vehicle- and metformin-treated K-Ctrl or K- Setd2KO mice. Individual tumor sizes normalized to the mean area of vehicle-treated control mice. Data represent the mean ± standard deviation. Data points represent individual tumors (K-Ctrl: n = 115 vehicle, n = 236 metformin; K-Setd2KO: n = 193 vehicle, n = 503 metformin). Significance determined by unpaired Student’s t-test. f Representative scans of tumor-bearing lobes from K-Ctrl or K-Setd2KO mice treated with vehicle or metformin. g Quantification of cell proliferation by the percentage of p-H3 positive cells in K-Ctrl and K-Setd2KO mice given vehicle treatment or metformin. Data indicate the mean ± standard deviation. Data points represent individual patient tumors (K-Ctrl: n = 13 Vehicle, n = 14 Metformin; K-Setd2KO: n = 14 Vehicle, n = 13 Metformin). Significance determined by unpaired Student’s t-test. Note: No survival experiments were conducted and efficacy of drug treatments is solely based on the differences in tumor size shown here.
Discussion
Here, we identify a conserved enhancement of gene expression programs and functional markers associated with oxidative metabolism and protein synthesis in SETD2-deficient cancers. The tumor suppressive function of the chromatin modifier SETD2, at least in the context of KRAS-driven lung adenocarcinoma, is therefore to limit pro-proliferative metabolic pathways by restricting oxidative metabolism and protein synthesis. As such, our data bolster the expanding realization that the metabolic state of a cell is intimately linked to the repertoire of post translational modifications on histones41,42,43.
Our work uncovers a role for SETD2 in constraining mitochondrial OXPHOS and mTORC1 signaling to limit cellular proliferation in the context of KRAS-driven lung adenocarcinoma. It is notable that these roles are not entirely dissimilar to those regulated by the LKB1 tumor suppressor, a well-known regulator of nutrient-sensing mechanisms that impinge on mTORC1 signaling44. Further, silencing or inactivation of Lkb1 also sensitizes lymphoma and lung adenocarcinoma to phenformin35,45. The parallels between the consequences of SETD2 and LKB1 inactivation are all the more provocative given an apparent functional-genetic epistatic relationship in KRAS-driven mouse models and their mutually exclusive pattern of mutation in human of lung adenocarcinoma patients2,15. Determining whether and how LKB1 and SETD2 fit into a singular pathway predicted by these overlapping phenotypes and their genetic epistasis is an intriguing possibility that has yet to be determined.
Finally, we demonstrate not only that signaling through OXPHOS and mTORC1 is required for the proliferative benefit bestowed upon tumor cells following SETD2 inactivation, but that they also represent readily actionable therapeutic vulnerabilities for patients with SETD2-deficient tumors. Therefore, our study nominates OXPHOS and mTORC1 inhibition as a targeted therapy for SETD2-deficient lung adenocarcinoma.
Methods
Animal studies and treatment
Animal studies were performed under strict compliance with Institutional Animal Care and Use Committee at University of Pennsylvania (804774). KrasLSL-G12D mice (Jax stock number 008179), Trp53flox/flox mice (Jax stock number 008462), and Rosa26LSL-YFP/ LSL-YFP mice have previously been described21,46,47. Mice are mixed B6J/129S4vJae. Mice were transduced with 6×104 plaque forming units (PFUs) per mouse of LentiCRISPRv2Cre by endotracheal intubation48. LentiCRISPRv2Cre expressing sgRNAs targeting GFP or β-Galactosidase (BGal) were used as controls, while an sgRNA targeting Setd2 was used for knockouts. For control sgRNAs, sgGFP was used to induce K tumors for RNA-sequencing, while sgBGal was used for all other experiments. The sgRNA sequences are: sgGFP-GGGCGAGGAGCTGTTCACCG, sgBGal – CACGTAGATACGTCTGCATC, and sgSetd2-AATGGGCTGAGGTACGCCGT14,49. Lentivirus production and titration was performed as described previously14.
For drug experiments, OpenStandard Diet was formulated with Rapamycin (MedChem Express) at 15 mg kg−1 for a dose of 2 mg/kg/day or IACS-10759 (MedChem Express) at 37.5 mg kg−1 for a dose of 5 mg/kg/day by Research Diets. Mice were placed on treatment diet 4 weeks prior to analysis. For phenformin treatment, mice were given the drug by oral gavage daily on a 5 days on/2 days off schedule for 4 weeks. Mice were given 200 mg/ kg/day phenformin dissolved in water (Cayman Chemicals). For metformin treatment, metformin was placed in the drinking water of mice at 1.25 mg/ml for 12 weeks prior to sacrifice (Sigma-Aldrich). No statistical methods were used to predetermine sample sizes. The size of each animal cohort was determined by estimating biologically relevant effect sizes between control and treated groups and then using the minimum number of animals that could reveal statistical significance using the indicated tests of significance. All animal studies were randomized in ‘control’ or ‘treated’ groups, and roughly equal proportions of male and female animals were used. However, all animals housed within the same cage were generally placed within the same treatment group. For histopathological assessments of tumor size, researchers were blinded to sample identity and group. The animal protocol was approved by the University Laboratory Animal Resources (ULAR) at the University of Pennsylvania and the IACUC.
Immunohistochemistry and immunofluorescence
Lung and tumor tissues were dissected into 10% neutral-buffered formalin overnight at room temperature before dehydration in a graded alcohol series. Paraffin-embedded and H&E-stained histological sections were produced by the Penn Molecular Pathology and Imaging Core. Immunostaining for H3K36me3 (Abcam, ab9050, 1:1000), p-4E-BP1(T37/46) (Cell Signaling Technology, cs2855, 1:100), mTOR (Cell Signaling Technology, cs2983, 1:100), LAMP2 (Abcam, ab13524, 1:100) and p-H3 (Cell Signaling Technology, cs9701, 1:500) were performed after citrate-based antigen retrieval. H3K36me3 alone was assessed by immunohistochemistry using ABC reagent (Vector Laboratories, PK-4001) and ImmPACT DAB (Vector Laboratories, SK-4105) according to the product instructions. P-4E-BP1(T37/46) was assessed by immunofluorescence using a biotinylated secondary antibody (Vector Laboratories, PK-4001) according to product instructions, and Streptavidin-conjugated Alexa594 (Thermo Fisher S11227, 1:200). Colocalization of mTOR and LAMP2 was determined using an anti-Rat Alexa647 antibody (Thermo Fisher A21247, 1:200) to detect LAMP2, and a biotinylated anti-Rabbit secondary antibody (Vector Laboratories, PK-4001) followed by Streptavidin-conjugated Alexa488 (Thermo Fisher S32354, 1:200) to detect mTOR.
Immunohistochemistry and immunofluorescence were both performed on paraffin-embedded sections following the same antigen-retrieval protocol. Sections were incubated in primary antibody overnight at 4 °C, secondary antibody for 1 hour at room temperature, and for immunofluorescence Streptavidin-conjugated fluorophore for 1 hour at room temperature in the dark.
For TUNEL staining, tissues were deparaffinized and then permeabilized with 0.1% sodium citrate and 0.1% Triton-X in PBS for 8 minutes. FITC-conjugated TUNEL labeling mix (Millipore Sigma, 11684795910) was added to permeabilized tissue sections and incubated for 1 hour at 37 °C in the dark. For all immunofluorescence staining, nuclei were stained using 5 mg/ml DAPI at a 1:1000 dilution for 10 minutes, and then slides were mounted with Fluoro-Gel (EMS, 17985-50).
O-Propargyl-Puromycin Analysis
For the quantification of protein translation, mice were injected intraperitoneally (IP) with 200 μl of a 10 mM solution of OP-Puro dissolved in PBS as previously described24. 1 hour after OP-Puro IP injection, mice were sacrificed and lungs were formalin fixed and paraffin embedded as described above. Co-immunofluorescence for H3K36me3 and OP-Puro was performed to quantify translation rates in tumors lacking SETD2 activity. Antigen retrieval was performed using a solution of 20 μg/ml proteinase K in TE Buffer (pH 8) at 37 °C for 10 minutes. A click chemistry reaction was then performed for 30 minutes at room temperature in the dark to conjugate Alexa594 to incorporated OP-Puro according to product instructions (Thermo Fisher, C10429, beginning at Step 5.1). Samples were kept in the dark for all further steps. Samples were treated with avidin and biotin blocking steps for 20 minutes each (Vector Laboratories, SP-2001), and a 30-minute protein block (Dako, X090930-2) before incubating with H3K36me3 primary antibody (Abcam, ab9050) at 1:300 overnight at 4 °C. H3K36me3 was then detected using a biotinylated secondary antibody for 1 hour (Vector Laboratories, PK-4001) followed by streptavidin-conjugated Alexa488 for 1 hour at a 1:200 dilution (Thermo Fisher, S-32354). Nuclei were stained using 5 mg/ml DAPI at a 1:1000 dilution for 10 minutes, and then slides were mounted with Fluoro-Gel (EMS, 17985-50).
Histological quantification
The analysis of mTOR/LAMP2 colocalization was performed using a Leica TCS SP5 II confocal microscope. Z-stack projections of confocal images taken of control and SETD2-deficient tumors were analyzed. For the quantification of PLA individual loci were counted from z-stack projections. The LAS X colocalization tool was used for the quantification of mTOR/LAMP2 colocalization, All other photomicrographs were captured on a Leica DMI6000B inverted light and fluorescence microscope, and ImageJ software was used for subsequent histological quantifications50. For the quantification of staining intensity for OP-Puro and p-4EBP1(T37/46), single fluorescent channel images were obtained and the mean fluorescence intensity of staining for each tumor was quantified in ImageJ. Great care was made to ensure that background signal from blood vessels, or empty spaces were excluded from the analysis. For the quantification of tumor sizes under varying conditions including drug treatments, a tile scan of each mouse lung was obtained using the Leica DMI600B microscope and tumor area was measured in ImageJ. Tumor areas were then normalized to the mean area of a sgCtrl, vehicle treatment tumor. For the quantification of p-H3 staining, total nuclei in each tumor were counted using the IHC Profiler plugin for ImageJ, and p-H3-expressing nuclei were counted in ImageJ using the Cell Counter plugin51. For all histological analyses each data point represents an individual tumor.
Flow Cytometry
Tumors were microdissected directly from the lungs of KrasLSL-G12D/+;Trp53flox/flox; Rosa26LSL-YFP/LSL-YFP (KPY) mice and individually placed in 500 μl of tumor digestion buffer consisting of PBS containing 10 mM HEPES pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 1.8 mM CaCl2, along with freshly added Collagenase 4 (Worthington 100 mg/ml solution, 20 μl per ml of digestion buffer) and DNase I (Roche 10 mg/ml solution, 4 μl per ml of digestion buffer). Tumors were manually disassociated using scissors, and then placed in a 4 °C shaker for 1 hour at 250 rpm. Digested tumors were then filtered into strainer-cap flow tubes (Corning, 352235) containing 1 ml of horse serum (Thermo Fisher, 16050122) to quench the digestion reaction. Cells were spun down at 200 g for 5 minutes with the cap in place to obtain all cells. The supernatant was aspirated, cells were washed once with PBS and then resuspended with a given mitochondrial dye to stain for 30 minutes at 37 °C. To quantify mitochondrial volume, cells were incubated with 50 nM MitoTracker Deep Red FM (Thermo Fisher, M22426), combined with 50 μM of CCCP (Thermo Fisher, M34151) to eliminate confounding effects of mitochondrial membrane potential differences. To quantify mitochondrial membrane potential cells were incubated with 20 nM MitoProbe DiIC1(5) (Thermo Fisher, M34151). To quantify mitochondrial oxidative potential cells were incubated with 100 nM MitoTracker Red CM-H2XRos (Thermo Fisher, M7513). To quantify mitochondrial ROS cells were incubated with 5 μM MitoSOX Red (Thermo Fisher, M36008). After 30 minutes of staining, cells were washed twice with PBS and then resuspended in 100 μl of staining solution of FACS buffer containing biotinylated antibodies against CD31 (BD Biosciences, 558737, 1:100), CD45 (BD Biosciences, 553078, 1:200), and Ter-119 (BD Biosciences, 553672, 1:100), for 25 minutes at a 4 °C. Cells were washed twice and resuspended in 100 μl of streptavidin-conjugated APC- eFluor 780 (Thermo Fisher, 47-4317-82) for 20 minutes. Finally, cells were washed twice and resuspended in FACS buffer containing DAPI at a 1:1000 dilution. Flow cytometry was performed using an Attune NxT flow cytometer (Thermo Fisher), and gating was performed to exclude doublets, dead cells, YFP- cells and non-epithelial contaminating cell types (see Supplementary Fig. 2c). Mitochondrial properties were then quantified by measuring the median fluorescence intensity of a given dye in live, YFP + tumor cells. For the quantification of YFP protein expression the mean fluorescence intensity of YFP was quantified for each sample by flow cytometry. For all flow cytometry analyses each data point represents an individual tumor.
RNA sequencing and human dataset analysis
For gene expression analysis in human tumors, RNA-sequencing data was obtained from the Cancer Genome Atlas lung adenocarcinoma dataset2. Due to the relative infrequency of SETD2 mutations, a comparison of mutant and wildtype cases had insufficient statistical power to draw meaningful conclusions. Therefore, mRNA expression data was extracted by the Penn Institute for Biomedical Informatics, and a pearson correlation score was calculated comparing the expression of SETD2 to all other genes. All genes were ranked in order according to the genes most negatively correlated with SETD2 expression to most positively correlated, and this rank list was used to perform Gene Set Enrichment Analysis (GSEA) examining GO biological processes using the molecular signature database (MSigDB)52,53. For gene expression analysis in K-Ctrl and K-Setd2KO tumors, analysis was performed on previous sequencing results14. For gene expression analysis in KPY-Ctrl and KPY-Setd2KO tumors, tumors were microdissected away from normal lung tissue, and digested into a single cell suspension as described above. Live, YFP + tumor cells were isolated by cell sorting, spun down and flash frozen in liquid nitrogen. RNA was extracted using the RNeasy Plus Micro kit (Qiagen, Catalog #74034) using 350 μl of RLT Plus and the QIAshredder columns as per manufacturer’s instructions. Total RNA quantity was measured using the Qubit RNA HS assay kit (ThermoFisher, Catalog #Q32852) and RNA quality was measured using a BioAnalyzer RNA 6000 Nano assay (Agilent, Catalog #5067-1511). Sequencing libraries were prepared on the Illumina NeoPrep and subjected to 75-bp single-end sequencing on the Illumina NextSeq 500 platform. Fastq files for each sample were aligned against the mouse genome, build GRCm38.p5, using Salmon (v0.8.2)54.
Differentially expressed genes were identified with DESeq2 (v1.17.0) and ranked according to the Stat value which considers both the significance, fold-change and directionality of the gene expression change (Supplementary Data 1)55. This rank of genes most upregulated upon Setd2 loss was then used to perform GSEA examining GO biological processes using the MSigDB. GSEA network plots of the top 20 pathways negatively correlated with SETD2 expression in both human and mice were then generated as previously described23. A graphical depiction of the network plot was then generated using Gephi v.0.9.2, and gene sets were characterized according to their functions56. To quantify YFP RNA expression the total YFP read count was quantified from RNA- sequencing data of KPY tumors using Salmon (v0.8.2).
To analyze 4E-BP1 protein levels and phosphorylation in human tumors, level 4 RPPA data from human lung adenocarcinomas was extracted from the Cancer Proteome Atlas website30,31. RPPA z-scores were matched with gene expression and genetic information from each sample represented in the TCGA lung adenocarcinoma dataset2. The RPPA z-score for phosphorylated 4E-BP1(T70) was compared to SETD2 mRNA expression z-scores for each sample. The levels of total 4E-BP1 and phosphorylated 4E-BP1(T70) were also compared between tumor samples with wildtype SETD2, and SETD2 deficiency (defined as tumors containing either an inactivating mutation in SETD2, homozygous loss of the gene, or a loss of 1 copy of SETD2 along with a mRNA z-score < −0.5).
Electron Microscopy
Tumors were microdissected directly from the lungs of mice and then the tissue was divided in half. One portion of each tumor was fixed for IHC to determine the H3K36me3-status of the given tumor as described above, while the other portion was fixed overnight in an osmium solution obtained from the Penn Electron Microscopy Resource Lab (EMRL), and then submitted to the EMRL for further tissue processing and staining with uranyl acetate and lead citrate. Transmission electron microscopy (TEM) was then performed using a JEOL JEM-1010 for both control and SETD2- deficient tumor samples. Images were taken at 60,000 to 150,000 X magnification, and mitochondrial properties were then quantified using ImageJ, normalizing to the magnification of the image. Mitochondrial size was in Image J, while mitochondrial number was quantified by counting the number of mitochondria per field of view across multiple 15,000 X magnification images. Mitochondrial cristae width was quantified in Image J by drawing a perpendicular line between the inner membranes of cristae, and then quantifying the resulting distance. Mitochondrial electron density was quantified by measuring the mean pixel darkness of the mitochondrial matrix in ImageJ and normalizing this to the mean pixel darkness of the surrounding cytoplasm. Mitochondrial cristae density was quantified by counting the number of cristae in an individual mitochondrion and then dividing by the mitochondrial area.
Cell lines
SETD2 shRNA sequences from Skutcha et al.57 were cloned into the retroviral MLP shRNA expression vector. shRNA sequences are: shSETD2 #1: CAAGCAAAGAAGTATTCAGAA and shSETD2 #2: CAACCAACAGTCTGTCAGTGT. NCI-H2009 human lung adenocarcinoma cells (from NCI cell line repository) were infected with retrovirus harboring shSETD2 #1, shSETD2 #2, or MLP empty vector. Antibiotic selection was performed and efficiency of SETD2 knockdown was assessed via immunoblot analysis and RT-PCR.
Immunoblot analysis
For whole cell lysates, cells were lysed in RIPA buffer. Acid-extracted histones were prepared for histone methylation Western blot. Samples were resolved on NuPage 4–12% Bis-Tris protein gels (Thermo Fisher) and transferred to polyvinylidene fluoride (PVDF) membranes. Blocking, primary and secondary antibody incubations were performed in Tris-buffered saline (TBS) with 0.1% Tween-20. H3K36me3 (1:1000, Abcam, ab9050), SETD2 (1:1000, Cell Signaling Technology, E4W8Q), H3 (1:10000, Abcam ab1791), β-actin (1:10000,Sigma Aldrich, A2066), were assessed by western blotting. β-actin and H3 were used as loading controls. Protein concentration was determined using a BCA protein assay kit (Thermo Fisher Scientific, 23225).
Quantitative reverse transcription-PCR
Total RNA was extracted from cells using Qiagen RNeasy Mini Kit (Qiagen, 74106). cDNA synthesis was performed using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, 4368814). RT-PCR was performed using SYBR Green I Nucleic Acid Gel Stain (Invitrogen, S7563) in triplicate, following manufacturer instructions, and evaluated on an Applied Biosystems ViiA 7 RT-PCR machine. Setd2 forward primer: CTTCTACCACGTATCAGCAACC, Setd2 reverse primer: GTAATCACGTGTCCCACCATAC. β-actin forward primer: CCAACCGCGAGAAGATGA, β-actin reverse primer: CCAGAGGCGTACAGGGATAG.
Seahorse XF Cell Mito Stress Analysis
Oxidative respiration was measured using XF Cell Mito Stress Test Kit (Agilent Technologies, 103015-100). 1 × 104 cells per well were seeded on an XF96 Cell Culture Microplate. Microplate was incubated for 24 h at 37 C. Seahorse XF96 FluxPak sensor cartridge was hydrated with 200 μl of Seahorse Calibrant in a non-CO2 incubator at 37 C overnight. After 24 h, cells were incubated with base medium (Agilent Technologies, 102353-100) containing 2 mM L-glutamine, 1 mM sodium pyruvate, and 10 mM glucose in a non-CO2 incubator at 37 C for 45 min prior to assay. Oxygen consumption rate (OCR) was measured by XFe96 extracellular flux analyzer with sequential injections of 1 μM oligomycin, 1 μM FCCP, and 0.5 μM rotenone/antimycin A. After the run, cells were lysed with 15 μl RIPA buffer and protein concentration was quantified using Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, 23225). OCR measurements were normalized to the protein concentration in each well.
Statistics and Reproducibility
All analyses were performed using Graphpad Prism (v.8.1.1). For all analyses of mitochondrial properties, immunofluorescence, percentage of p-H3+ cells, normalized tumor areas and RPPA analysis comparing SETD2 wildtype and deficient tumors, unpaired Student’s t-tests were performed. For the comparison of SETD2 mRNA expression and p-4E-BP1(T70) levels by RPPA, a linear regression analysis was performed. Outliers were excluded from rapamycin, IACS-10759 and phenformin experiments using the ROUT method with a Q of 0.1%. Sample sizes for individual experiments are indicated in the figure legends. Reproducibility of the findings were confirmed by analyzing at least n = 3 technical and/or independent biological replicates as indicated in the figure legends. The findings of all the biological replicates were consistent.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw RNA sequencing data associated with Fig. 1b–e and Supplementary Fig. 1h, i have been deposited publicly in the Gene Expression Omnibus (GEO) under accession number GSE224270. The list of differentially expressed genes associated with Fig. 1b–e and Supplementary Fig. 1h, i are available in Supplementary Data 1. Source data associated with Figs. 2b–I, 3b, d, f, 4b, d, e, g and Supplementary Fig. 2b, d, e, 3b, d, and 4a–d are available in Supplementary Data 2. Uncropped western blots are available in Supplementary Fig. 6.
References
Kandoth, C. et al. Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339 (2013).
Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 511, 543–550 (2014).
Zehir, A. et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23, 703–713 (2017).
Edmunds, J. W., Mahadevan, L. C. & Clayton, A. L. Dynamic histone H3 methylation during gene induction: HYPB/Setd2 mediates all H3K36 trimethylation. EMBO J 27, 406–420 (2008).
Park, I. Y. et al. Dual Chromatin and Cytoskeletal Remodeling by SETD2. Cell 166, 950–962 (2016).
Chen, K. et al. Methyltransferase SETD2-Mediated Methylation of STAT1 Is Critical for Interferon Antiviral Activity. Cell 170, 492–506 (2017). e414.
Yuan, H. et al. SETD2 Restricts Prostate Cancer Metastasis by Integrating EZH2 and AMPK Signaling Pathways. Cancer Cell 38, 350–365 (2020). e357.
Dhayalan, A. et al. The Dnmt3a PWWP domain reads histone 3 lysine 36 trimethylation and guides DNA methylation. J Biol Chem 285, 26114–26120 (2010).
Naftelberg, S., Schor, I. E., Ast, G. & Kornblihtt, A. R. Regulation of alternative splicing through coupling with transcription and chromatin structure. Annu Rev Biochem 84, 165–198 (2015).
Li, J. et al. SETD2: an epigenetic modifier with tumor suppressor functionality. Oncotarget 7, 50719–50734 (2016).
Fahey, C. C. & Davis, I. J. SETting the Stage for Cancer Development: SETD2 and the Consequences of Lost Methylation. Cold Spring Harb Perspect Med 7, a026468 (2017).
Huang, H. et al. Histone H3 trimethylation at lysine 36 guides m(6)A RNA modification co-transcriptionally. Nature 567, 414–419 (2019).
Rogers, Z. N. et al. A quantitative and multiplexed approach to uncover the fitness landscape of tumor suppression in vivo. Nat Methods 14, 737–742 (2017).
Walter, D. M. et al. Systematic In Vivo Inactivation of Chromatin-Regulating Enzymes Identifies Setd2 as a Potent Tumor Suppressor in Lung Adenocarcinoma. Cancer Res 77, 1719–1729 (2017).
Rogers, Z. N. et al. Mapping the in vivo fitness landscape of lung adenocarcinoma tumor suppression in mice. Nat Genet 50, 483–486 (2018).
Feldser, D. M. et al. Stage-specific sensitivity to p53 restoration during lung cancer progression. Nature 468, 572–575 (2010).
Walter, D. M. et al. RB constrains lineage fidelity and multiple stages of tumour progression and metastasis. Nature 569, 423–427 (2019).
Cogliati, S. et al. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 155, 160–171 (2013).
Cogliati, S., Enriquez, J. A. & Scorrano, L. Mitochondrial Cristae: Where Beauty Meets Functionality. Trends in Biochemical Sciences 41, 261–273 (2016).
Nielsen, J. et al. Plasticity in mitochondrial cristae density allows metabolic capacity modulation in human skeletal muscle. The Journal of Physiology 595, 2839–2847 (2017).
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1, 4 (2001).
Mitsudomi, T. et al. p53 gene mutations in non-small-cell lung cancer cell lines and their correlation with the presence of ras mutations and clinical features. Oncogene 7, 171–180 (1992).
Rasool, R. U. et al. CDK7 Inhibition Suppresses Castration-Resistant Prostate Cancer through MED1 Inactivation. Cancer Discov 9, 1538–1555 (2019).
Liu, J., Xu, Y., Stoleru, D. & Salic, A. Imaging protein synthesis in cells and tissues with an alkyne analog of puromycin. Proc Natl Acad Sci U S A 109, 413–418 (2012).
Signer, R. A., Magee, J. A., Salic, A. & Morrison, S. J. Haematopoietic stem cells require a highly regulated protein synthesis rate. Nature 509, 49–54 (2014).
Iwasaki, S. & Ingolia, N. T. The Growing Toolbox for Protein Synthesis Studies. Trends Biochem Sci 42, 612–624 (2017).
Cunningham, J. T. et al. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature 450, 736–740 (2007).
Saxton, R. A. & Sabatini, D. M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 168, 960–976 (2017).
de la Cruz López, K. G., Toledo Guzmán, M. E., Sánchez, E. O. & García Carrancá, A. mTORC1 as a Regulator of Mitochondrial Functions and a Therapeutic Target in Cancer. Frontiers in Oncology 9, 1373 (2019).
Li, J. et al. TCPA: a resource for cancer functional proteomics data. Nat Methods 10, 1046–1047 (2013).
Li, J. et al. Explore, Visualize, and Analyze Functional Cancer Proteomic Data Using the Cancer Proteome Atlas. Cancer Res 77, e51–e54 (2017).
Gingras, A. C. et al. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev 15, 2852–2864 (2001).
Qin, X., Jiang, B. & Zhang, Y. 4E-BP1, a multifactor regulated multifunctional protein. Cell cycle (Georgetown, Tex.) 15, 781–786 (2016).
Dowling, R. J. O. et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science (New York, N.Y.) 328, 1172–1176 (2010).
Shackelford, D. B. et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell 23, 143–158 (2013).
Xie, J., Wang, X. & Proud, C. G. mTOR inhibitors in cancer therapy. F1000Res 5, (2016).
Molina, J. R. et al. An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat Med 24, 1036–1046 (2018).
Misbin, R. I. The Phantom of Lactic Acidosis due to Metformin in Patients With Diabetes. Diabetes Care 27, 1791 (2004).
Lee, M.-S. et al. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer 11, 20 (2011).
Currie, C. J. et al. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 35, 299–304 (2012).
Carrer, A. & Wellen, K. E. Metabolism and epigenetics: a link cancer cells exploit. Curr Opin Biotechnol 34, 23–29 (2015).
Reid, M. A., Dai, Z. & Locasale, J. W. The impact of cellular metabolism on chromatin dynamics and epigenetics. Nat Cell Biol 19, 1298–1306 (2017).
Ye, C. & Tu, B. P. Sink into the Epigenome: Histones as Repositories That Influence Cellular Metabolism. Trends Endocrinol Metab 29, 626–637 (2018).
Herzig, S. & Shaw, R. J. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol 19, 121–135 (2018).
Izreig, S. et al. Repression of LKB1 by miR-17 approximately 92 Sensitizes MYC-Dependent Lymphoma to Biguanide Treatment. Cell Rep Med 1, 100014 (2020).
Jackson, E. L. et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev 15, 3243–3248 (2001).
Jackson, E. L. et al. The differential effects of mutant p53 alleles on advanced murine lung cancer. Cancer Res 65, 10280–10288 (2005).
DuPage, M., Dooley, A. L. & Jacks, T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protoc 4, 1064–1072 (2009).
Shalem, O. et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9, 671–675 (2012).
Varghese, F., Bukhari, A. B., Malhotra, R. & De, A. IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One 9, e96801 (2014).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102, 15545–15550 (2005).
Liberzon, A. et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics 27, 1739–1740 (2011).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods 14, 417–419 (2017).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550 (2014).
Bastian, M., Heymann, S. & Jacomy, M. Gephi: An Open Source Software for Exploring and Manipulating Networks. ICWSM 8, 361–362 (2009).
Skucha, A. et al. MLL-fusion-driven leukemia requires SETD2 to safeguard genomic integrity. Nat Commun 9, 1983 (2018).
Acknowledgements
We would like to thank ULAR staff for animal husbandry, the Molecular Pathology and Imaging Core (MPIC) for histological analysis, B. Zuo for help with electron microscopy, A. Bedenbaugh for tissue processing, A. Durham and E. Radaelli for help with pathology, Bang-Jin Kim for assistance with confocal microscopy, N. Anderson for help with mitochondrial stress test assay, and M. Winslow, K. Wellen, and S. Zhao for helpful discussions and critical reading of the manuscript. This work is supported by: NIH grants (R01-CA262619 and R01-CA222503 to D.M.F., 2-T32-CA-15299-15 to A.C.G.) and Department of Defense grant (LCD400095 to D.M.F.).
Author information
Authors and Affiliations
Contributions
D.M.W. and A.A.G. performed animal studies. D.M.W., R.N., and A.C.G. performed bioinformatics analyses with supervision from I.A.A. and D.M.F. A.C.G. performed cell culture studies. D.M.W., K.R.D., K.M.A., and J.O.A. performed histopathological analyses of mouse specimens. D.M.W. conducted electron microscopy analysis with supervision from D.C.W. D.M.W., A.C.G., K.R.D., S.G.B., and D.M.F. interpreted all datasets. D.M.W. and A.C.G. drafted portions of the manuscript. D.M.F. conceived and designed the project, and wrote the manuscript with editorial help from D.M.W. and A.C.G.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
: Communications Biology thanks Albert Jeltsch, Fuchun Yang and the other, anonymous, reviewer for their contribution to the peer review of this work. Primary Handling Editors: Marina Holz and Zhijuan Qiu. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Walter, D.M., Gladstein, A.C., Doerig, K.R. et al. Setd2 inactivation sensitizes lung adenocarcinoma to inhibitors of oxidative respiration and mTORC1 signaling. Commun Biol 6, 255 (2023). https://doi.org/10.1038/s42003-023-04618-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-04618-3
This article is cited by
-
Tumor-suppressive functions of protein lysine methyltransferases
Experimental & Molecular Medicine (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.