Abstract
Termites (Blattodea: Isoptera) have evolved specialized defensive strategies for colony protection. Alarm communication enables workers to escape threats while soldiers are recruited to the source of disturbance. Here, we study the vibroacoustic and chemical alarm communication in the wood roach Cryptocercus and in 20 termite species including seven of the nine termite families, all life-types, and all feeding and nesting habits. Our multidisciplinary approach shows that vibratory alarm signals represent an ethological synapomorphy of termites and Cryptocercus. In contrast, chemical alarms have evolved independently in several cockroach groups and at least twice in termites. Vibroacoustic alarm signaling patterns are the most complex in Neoisoptera, in which they are often combined with chemical signals. The alarm characters correlate to phylogenetic position, food type and hardness, foraging area size, and nesting habits. Overall, species of Neoisoptera have developed the most sophisticated communication system amongst termites, potentially contributing to their ecological success.
Similar content being viewed by others
Introduction
To ensure communal living, the existence of a common defense among group members and against any threat is essential. Defensive strategies range from construction of protective barriers to coordinated responses to a particular threat, a predator, competitor, or pathogen. These responses require complex coordination, either in behavioral repertoires or physiological responses. In addition, they may be associated with concomitant morphological specializations which are obtained by building on existing or coopting new developmental pathways to achieve such functions. In essence, a successful defense, whether of a single mother defending her brood or a colony of millions, is the result of a plethora of evolutionary changes honed to increase the collective survivorship of the individuals participating in communal life1,2.
In contrast to solitary animals, which can only rely upon themselves to protect against predators, some animals in social groups may dedicate themselves exclusively to foraging under the protection of specialized conspecifics. In the case of danger, the latter alert the former by alarm signaling. Such task partitioning allows the group to be most efficient at low risk3. This kind of communication amongst conspecifics is called alarm communication or alarm signaling. It is a defensive strategy that has evolved independently in many social animals, either vertebrates or arthropods, as it increases the fitness of social groups4,5,6,7,8,9,10,11,12. An alarm signal emitted by a colony member can make conspecifics aware of danger13. Thus, such a signal can be used by nearby conspecifics to rapidly respond to by displaying defensive or evading behaviors in order to prevent or limit casualties14. Usually, the first animal alarm signals that come to mind are the familiar alarm calls. These acoustic signals are common in mammals and birds4,15, such as the classic example of the alarm calls in Vervet monkeys16.
Alarm signals may also be transmitted in many animals by two distinct sensory channels: vibroacoustic and chemical17,18. Vibroacoustic communication involves substrate- and/or air-borne vibrations. It is considered as the most ancient and taxonomically widespread form of communication19, and more than 90% of insects use substrate-borne vibrations alone or in concert with other forms of signaling20. In eusocial insects, the vibroacoustic signals act as either short-range (tactile) signals21,22,23, or long-range vibrations perceived by distant nestmates through the Johnston’s (air-borne) or subgenual (substrate-borne) organs24,25. Vibroacoustic signaling may carry various messages, such as alarm, recruitment, or begging for food23,26,27,28. Specific means of vibroacoustic communication were observed in termites but not in other social insects: alarming nestmates in response to pathogen encounter29, evaluation of volume of the remaining wood30 or perception of approaching competitor31. Apart of stridulatory organs in many ant groups, vibroacoustic signals are generated by inconspicuous body parts showing little to no specialization to this particular task23.
Chemical alarm signals are widespread in animals, including insects17,32,33. The release of a volatile substance, an alarm pheromone, warns conspecifics of danger. Alarm pheromones provoke strong dose- and context-specific responses, resulting in retreat or attack, the latter usually accompanied with fast changes in caste or age-category proportions of the insects involved34,35,36,37. While the glandular origins of the alarm pheromones are diverse and taxon-specific37,38,39,40, it is important to note that all alarm pheromones are produced by abdominal glands in cockroaches, while exclusively by cephalic glands in termite soldiers27,34,41,42.
All social insects have evolved complex defensive traits, including morphological adaptations, chemical defenses, structural nest modifications, and alarm behaviors, as inherent components of colony defense43,44,45,46. Both vibroacoustic and chemical signals occur in all major eusocial insect groups (termites, ants, bees, and wasps). These signals are responsible for the alarm behavior, defined for termites by Deligne and coauthors47 as a specific behavior implying the emission of a signal by an individual that has experienced a dangerous situation, the perception of this signal by distant nestmates, and subsequent adaptive modifications in their behavior. Such modifications consist of a general increase in the level of activity, locomotive changes, aggregation, flight, and recruitment of other termites to the site of the disturbance. Thus, the emergence of specialized defensive castes and complex behaviors34,41,47 presumably contributed to the ecological success of termites and led to their extraordinary abundance throughout the tropics11,12,47,48,49,50.
Unlike social Hymenoptera, termites are hemimetabolous insects and the foraging parties comprise juvenile individuals, which are wingless and largely unsclerotized51. The first line of termite defense is passive, and consists in the physical isolation of the colony from the hostile environment, built and maintained by vulnerable workers34. The active defense strategies are best exemplified by large soldiers (such as in Mastotermes, Macrotermes, Syntermes, Cornitermes, Labiotermes, or Cubitermes), which can inflict deep wounds with their mandibles, often coupled with the release of toxic or anti-healing compounds making termite bites truly unforgettable34,52,many personal observations from D.S.D. and J.Š. These toxins may be released in copious amounts, as is the case in Coptotermes, in which the frontal gland secretion represents over a third of soldier fresh body weight53. The sticky toxic and irritating content of the frontal gland is sprayed at a distance from the nasus of the soldiers in Nasutitermitinae47. Other strategies are as peculiar as closing entrance holes with soldier heads (phragmosis) or strikes by modified symmetrical or asymmetrical snapping mandibles causing devastating wounds to invertebrates34,41,54,55. Defense is not restricted to soldiers, the defensive mechanism of workers in Neocapritermes taracua involves self-sacrifice through body rupture, allowing two separately stored secretions to come into contact together and to produce a sticky and toxic cocktail harmful to opponents56,57.
In nature it is common to see individuals of some social insects warning their nestmates of potential danger. These direct observations repeatedly revealed the importance of alarm communication in many insects23,37,58. In termites, any disturbance triggers seemingly erratic movements leading to effective defensive responses34,35,36,59,60, and enhanced protection of the colony due to the increase of the soldiers-to-worker ratio at the place of disturbance. This strategy makes the whole group unpalatable even to specialized vertebrate predators61. Our understanding of the proximate mechanisms of alarm communication is still limited, and because termites, ants, bees, and wasps are known to respond to the threat stimuli in a context-dependent fashion, the acquisition of empirical data and their interpretation remains challenging62.
In spite of the crucial importance of alarm communication for termite colony survival, only fragmented reports have hitherto been published about this topic, most of which focused on either vibroacoustic or pheromonal communication of isolated species27,36,59,60,63,64,65,66,67,68,69,70,71. The evolutionary trajectories of alarm signals, and their significance within complex ecological constraints across extant termite lineages, have not previously been investigated, and there is no report on alarm communication in soil-feeding termites, which represents over half of termite diversity70. In this work, we carried out a detailed study on nine termite species that, combined with existing knowledge on alarm communication in cockroaches and termites, includes members of all major lineages and ecological strategies (Table S1). We then studied the evolution of alarm characters in a phylogenetic context, and in relation to a series of social and ecological features, which are key factors influencing communication abilities in social animals58.
While vibroacoustic alarm signals evolved prior to achieving eusociality, the occurrence of alarm pheromones is linked to a wood-feeding habit and to populous colonies. These results indicate how important these ecological features are for the means of communication within termite colonies. In spite of the strong evolutionary signal, the influence of environment is best evidenced in the desert termite Hodotermes having lost alarm signaling as it lives in sandy soils and forages outside. The sophisticated alarm communication of termites may at least partially explain the ecological success of these eusocial insects in the tropics.
Terminology
We use the following definitions for the behaviors (all shown in Movie S1) observed in this study:
Locomotion speed is the average speed-of-motion of two individuals, either workers or soldiers, independently, per experiment, expressed in mm/s.
Burst is a sequence of oscillatory movements with stable spans between the beats at low or high frequency. It can be performed as a tremulation, drumming, or head-banging sequence.
Tremulation (body shaking) is the longitudinal oscillatory movements sensu Howse71, during which the head or the abdomen rarely hits the substrate. Tremulation signals are used at either low (≤15 Hz) or high frequency (>15 Hz), which we refer to as “low tremulations” and “high tremulations”, respectively.
Drumming is the vertical oscillatory movements sensu Howse71 during which the abdomen hits the substrate. Drumming is present in both workers and soldiers and is always displayed at high frequency (>15 Hz).
Head-banging is performed exclusively by soldiers hitting the substrate with their heads at high frequency (>15 Hz).
Results and discussion
General behavioral responses (avoidance and aggregation)
Wilson and Regnier38 classified the alarm responses in ants as either “panic” or “aggressive”. As the specific alarm signaling in termites (comparable to panic in ants) is a subtle behavior71,72 out of the scope of this work, general alarm is accompanied by a dramatic change in the group behavior. The general alarm typically involves many individuals disturbed at foraging sites, or present in a part of the nest that has been locally damaged27,36,59,60,63,64,65,66,67,68,69. The alerting termites search for quiet termites, touch them with their antennae, and perform tremulations to alert them36. The alarm responses usually result in a high soldier recruitment activity at disturbance locations, where soldiers displayed defensive postures, often combined with the release of defensive secretions produced by the labial glands during the opening/closing of mandibles26,27. Locomotion activity increased in many cases, especially in Reticulitermes, and disturbed workers usually displayed higher locomotion activity (evasion) than soldiers (defensive confrontation; Fig. 1 and Tables S1 and S2).
Locomotion speed was recorded separately for workers (n = 2) and soldiers (n = 2). The green fields indicate significantly different locomotion speed after stimulation, upward arrows mean the speed increased, downward arrows mean the speed decreased, the blue fields indicate no significant difference, NA means data not available.
Workers attempted escaping from the source of disturbance by moving away rapidly, while soldiers often searched for the source of disturbance and aggregated around it, resulting in a slower-motion patrolling behavior usually combined with scanning of the space with wide-spread antennae and mandibles ready to be triggered (when present). Soldiers of the majority of the studied species with biting-type mandibles started opening mandibles after direct disturbance because of two reasons: (i) open mandibles are prepared to bite once the opponent reaches the antennae, (ii) repeated openings of mandibles stimulate the release of defensive chemicals from cephalic glands as the mandibular muscles squeeze the liquids out of reservoirs. The defensive secretion is usually delivered to the opponent together with the bite34,47,54,73. The response to disturbance also included higher production of vibroacoustic alarm by disturbed individuals that warns nestmates (Table S3). Aside from such common behavioral responses, more specific actions were repeatedly observed. Workers and soldiers of Hodotermopsis frequently showed a type of drumming that substantially differs from that of all other termites—vigorous oscillatory movements against the lid of the Petri dish (not against the ground as in all other cases). While the increased locomotion was mainly observed in response to direct disturbance, there were a few exceptions, as in Neotermes and Termes, in which soldiers significantly decreased their locomotion speed, although such change was observed in Neotermes in long-term response only, implying that the patrolling behavior followed the active search for the source of disturbance. Finally, Hodotermes was a remarkable outlier in its general behavioral response to disturbances, as both castes stopped all movements after the disturbance for a short time (freezing behavior), but resumed their previous activity within 1–2 seconds. Thus, based on our experiments and repeated field observations, it is likely that the escaping behavior (higher locomotion speed) of workers and the aggregation of soldiers towards a disturbance is a basal trait to all extant termites that was secondarily lost only once, in Hodotermes (Fig. 1 and Table S1).
Alarm pheromones
Alarm pheromones in termites originate from soldiers’ defensive glands only: the labial glands in Mastotermes27 and the frontal gland in Neoisoptera (the derived group comprising Stylotermitidae, Rhinotermitidae, Serritermitidae, and Termitidae)36,59,63,64,65,67,68,69. Similar signals are widely used in some cockroaches, produced by the abdominal sternal or tergal glands (Eurycotis74; Therea75; Blaberus76). Benzoquinone, the alarm pheromone of Mastotermes, originates from the soldiers’ labial glands, and triggers a typical alarm behavior including caste-dependent change in locomotion speed and increased vibroacoustic signaling. All other alarm pheromones originate from the frontal gland, a termite-specific organ with no equivalent in other groups77. The frontal gland of soldiers is a saccular gland that opens to the exterior through the fontanelle in all Neoisoptera species we studied but Glossotermes, which has a blind-ended sac in the thorax and abdomen, whose contents can eventually be released through self-sacrifice via body rupture34,73.
Our study included three genera of Neoisoptera using alarm pheromones—Prorhinotermes, Reticulitermes, and Constrictotermes (Fig. 2 and Table S1), along with data from the literature. The active components of alarm pheromones are terpene hydrocarbons in all Neoisoptera34,41,68. Although Glossotermes increased the locomotion speed in response to a crushed soldier head, our chemical analyses did not reveal any alarm pheromone candidate (Fig. S1, Table S4), in line with the lack of a frontal gland reservoir in its head73. However, more species revealed responses to crushed heads devoid of defensive glands or conspicuous volatiles (see Fig. 1 and Table S1), possibly because colony members can perceive the smell of dead or wounded termites78,79,80,81. The two soil-feeding species we studied (Labiotermes and Termes) lack alarm pheromones, unlike wood-feeders that used alarm pheromones. In addition to field observations, it suggests that alarm pheromones are not used by the soil-feeding groups, representing altogether 60% of termite species diversity70. According to recent termite phylogenies82,83, alarm pheromones evolved at least twice; in the most basal extant termite clade, Mastotermitidae, and then in Neoisoptera, in which the lack of observations precludes our distinguishing between a single origin followed by multiple losses or multiple origins (Fig. 2). Our investigation suggested that the species’ life-type and related traits strongly correlated with the presence of alarm pheromones (Fig. S2), as species living either in small colonies (Archotermopsidae, Kalotermitidae70,84), or those living in open arid environments (Hodotermitidae70,84), lack this communication channel.
Vibroacoustic signaling
Termites generally responded to disturbance by violent shaking and drumming, sometimes accompanied by sounds audible to the observer (Movie S1). The beats were arranged into bursts of low frequency (under 15 Hz) in the case of low tremulation, or high frequency (above 15 Hz) high tremulation, drumming or head-banging. The vibroacoustic signature was specific to a given genus (Figs. 3, 4, and S3), a feature not previously recognized.
In contrary to Fig. 2, we included only species for which vibroacoustic communication is known in sufficient details.
The nymphs of the wood roach Cryptocercus and termite workers in black, and drumming by termite soldiers (in white). On each box, the central mark is the median, the edges of the box are the 25th and 75th percentiles, the whiskers extend to the most extreme datapoints the algorithm considers to be not outliers, and the outliers are plotted individually. Cryptocercus and Neotermes produced low frequency signals only. Hodotermes is not included as it does not communicate via body vibrations.
While the species descending from early diverging lineages (including Cryptocercus85) revealed a rather monotonous pattern of beats arranged into singular bursts, the patterns became more diverse in Neoisoptera, such as in Glossotermes, and especially in Termitidae, which use a combination of several bursts into a single vibroacoustic event (Figs. 3 and S3). The tremulations were primarily used as short-range communication to alert naïve nestmates, and carried relatively low energy compared to drumming or head-banging. The tremulations, when processed and amplified, are audible as muffled noise, while drumming and head-banging sounds like a series of sharp hits (Movie S1). The occurrences of respective signal components are summarized in Table S1. Workers and soldiers within a species mostly share the same repertoire, although additional signals, such as head-banging, occur in soldiers only. The general trends show that the larger species vibrate at lower frequencies, and Cryptocercus and Neotermes, both high above the average termite size, lack the high-frequency signals completely. Hodotermopsis is unique among termites for hitting its head against the ceiling for drumming, not the floor, in both workers and soldiers. Head-banging was abundantly recorded in Glossotermes and Reticulitermes (Figs. 3, 4, and S3), but was only rarely observed in Labiotermes. All Termitidae displayed relatively conserved and complex patterns of vibroacoustic sequences combining abdomen drumming, and high and low tremulations into long sequences (Movie S1, Figs. 3, and S3). Notably, vibroacoustic signals were extremely well conserved within species, with little variation among subsequent beats, showing that alarm signals have been quite stable since Cryptocercus and termites diverged (Figs. 5 and S4), an event dating back to at least the Late Jurassic82,83. We may therefore assume that the pre-social ancestor of Cryptocercus and termites used alarm signals of comparable precision.
The values are given as percentage difference (positive or negative) from the mean duration. On each box, the central mark is the median, the edges of the box are the 25th and 75th percentiles, the whiskers extend to the most extreme datapoints the algorithm considers to be not outliers, and the outliers are plotted individually.
Evolutionary trajectories of alarm signals in termites
Our ancestral-states reconstructions indicate a single origin of vibroacoustic alarm communication in the common ancestor of all termites and their sister group, the wood roach Cryptocercus (Fig. 2). Its loss in Hodotermes (and Anacanthotermes, D.S.D. and J.Š., field observation) is probably due to environmental conditions: nesting in soft sandy ground and randomly foraging in the open air to collect dry grass presumably prevents effective transmission of vibrations or odors. To our best knowledge, the loss of vibroacoustic alarms occurred exclusively in Hodotermitidae. Whether vibratory communication disappeared completely in this group, or whether it was partly retained in another social context such as nest defense, as it is common in other termites86,87,88,89, remains to be determined.
Even though vibroacoustic communication is shared by all colony members, the actual involvement of the different castes in alarm signaling has rarely been studied. Our data show that both castes mostly share identical part on the communication, except in Glossotermes, Reticulitermes, and Labiotermes, in which soldiers perform head-banging, a soldier-specific behavior. Apart of these differences, our data do not support increased involvement of soldiers in alarm signaling, in contradiction to observations made by Stuart90. We could observe increased diversity of alarm signals in derived termite taxa, evidenced mostly in vibroacoustic alarm sequences, since alarm pheromone data are made only in the presence/absence of this channel.
Multiple Correspondence Analysis (MCA) analysis suggested that several ecological characteristics have a strong influence upon vibroacoustic communication. The most prominent ecological characters are the hardness of the food and the nest material, which, along with phylogenetic position, are the most important features influencing vibroacoustic signaling (Figs. 6 and S5), probably because relatively hard substrates facilitate the transmission of such signals. In addition, we found a clear relationship between the frequency of oscillatory movements used in vibroacoustic communication and termite body size, since larger species always communicate at lower frequencies than smaller ones.
Both graphs are in fact the same, and the left one shows the most influential characteristics of vibro-acoustic features (in blue) to the MCA analysis and the right one shows the most important ecological characters (in green) that might have an effect on vibroacoustic traits. The larger the size of the letters, the more important the character.
Pairwise correlation analyses showed that vibroacoustic characters are strongly correlated between the soldier and worker castes. Moreover, it seems that the presence of tremulation correlates with drumming in both castes across all species, and that the presence of tremulation in the soldier caste is correlated with the size ratio between castes (Fig. S2). Termite taxa with proportionally large soldiers compared to workers, such as Hodotermopsis or Neotermes, primarily rely on soldier behavior for the spread of alarm, while termite taxa in which the soldier and worker castes have similar sizes rely equally on soldiers and workers to communicate. There is certainly a phylogenetic component to this as soldiers in more basal lineages tend to be larger, but it possibly also reflects the fact that heavier individuals spread vibroacoustic alarm more efficiently, rendering the spread of alarm communication by smaller individuals obsolete. Whether the specialization for alarm transmission of larger individuals holds or not among subcastes of species with polymorphic soldiers, such as some Macrotermitinae (Macrotermes, Acanthotermes, Ancistrotermes) or some Nasutitermitinae (Diversitermes, Trinervitermes), remains to be investigated.
An interesting correlation exists between the presence of chemical alarm and the colony life-type (Fig. S2). One-piece nesters living in hard wood (common among basal termites) do not utilize chemical alarms, probably because vibroacoustic alarm communication is sufficient in small colonies sheltered in sound wood84,91. These species usually defend against intruders at a few “bottleneck” entrance points in the gallery network92. Termite colonies contained within a small gallery system of a single piece of wood also use vibrations for purposes other than alarm communication. For example, Cryptotermes spp. may evaluate a looming resource shortage and perceive approaching competitors through substrate vibrations, which may result in the initiation of mass production of alates for a final dispersal flight30,31. Our data suggest that chemical alarms have only emerged in termite species that are able to use food resources outside their nest location, although not all central site-nesting termites appear to possess these. Species colonizing new food sources through underground foraging galleries are more likely to encounter enemies inside their galleries93, potentially increasing the selection pressure for the acquisition of an alarm pheromone. The use of volatile alarm pheromones may efficiently alert naïve individuals of a potential threat when they approach a disturbed area, while soft substrates may not favor vibroacoustic communication. We can speculate that alarm pheromones may persist in the air even after the death of the foraging termites, triggering local avoidance of the newcomers.
The identification of alarm pheromones is intricate, and therefore they have been identified for only a few species27,36,59,65,68,94. Here, we have not studied the chemical nature of alarm pheromones, but merely their presence/absence based on the soldier crushed heads, behavioral responses to them, and the composition of their volatiles. A maximum parsimony model supports independent evolutionary origins of chemical alarms in Mastotermitidae and Neoisoptera, followed by repeated losses in the latter lineage. Many termite and cockroach species produce defensive compounds, often irritating, that, presumably, can be co-opted as alarm pheromones, explaining the diversity of alarm pheromones and their glandular origins found among Blattodea under highly variable selective pressures27,34,74,75,76.
Conclusion and future directions
Alarm behaviors are ubiquitous in termites (excepting Hodotermitidae), confirming that caste-dependent responses to disturbances (workers primarily hide away while soldiers confront the threat) is a plesiomorphic characteristic of termites that later diversified with the rise of extant termite lineages. The use of vibroacoustic alarm signals evolved prior to the evolution of eusociality in termites, as their homologs are present in the wood roach Cryptocercus, indicating a shared origin in their most recent common ancestor. Subsequently, as termite lineages proliferated, the nature of vibratory signals became progressively more variable in Neoisoptera, with clear patterns of low and high tremulations, drumming, and head-banging. Alarm pheromones appeared in soldiers at least twice, from compounds secreted by the labial glands in Mastotermes, or from compounds derived from the frontal gland in Neoisoptera. However, while the soldier frontal gland was a major evolutionary innovation that likely contributed to the success of Neoisoptera48,70, the allomonal secretions may have gained the secondary function of an alarm signal in some clades only. The absence of alarm pheromones in Glossotermes therefore raises the possibility that the common ancestor of all modern Neoisoptera did not use an alarm pheromone, which, instead, first evolved later on, in more derived Rhinotermitidae, and was secondarily lost in some taxa (Labiotermes, Termes, etc.). The investigation of phylogenetically basal Neoisoptera (Stylotermitidae, Rhinotermitinae) is needed to confirm, or reject, this scenario. Although Mastotermes is the only non-Neoisoptera known to use an alarm pheromone, it is still possible that some other understudied taxa have acquired this chemical signal as well. For example, Paraneotermes simplicicornis is the only member of Kalotermitidae known to have the ability to nest underground and to forage for many wood items95,96, a trait we found strongly associated with the use of a chemical alarm. The investigation of such outliers may provide additional insights into the evolution of alarm behaviors, and into the ecological pressures driving them. Another interesting factor possibly influencing alarm communication is the presence of a soldier caste, which was lost at least three times independently in (i) Apicotermitinae, (ii) Orientotermes and Protohamitermes, and (iii) Invasitermes (all Termitidae97,98,99). Workers in these groups are fully responsible for colony defense, and they thus reveal high levels of agonism and sometimes also developed unique defense strategies, such as body rupturing34,47,100. Furthermore, the remarkable absence of alarm signals and responses to danger stimuli in Hodotermitidae underline the effect of ecological factors on the communication skills in a given species. The investigation of other termite species with various feeding, foraging, and nesting habits could therefore reveal novel defense mechanisms, including alarm communications that may have been selected for under different ecological pressures. Regardless, communication among individuals responding to distress evolved well prior to the eusocial system so characteristic of termite life and from vibroacoustic systems found widely among arthropods. Subsequently, chemical refinements to this communication system evolved multiple times and assuredly contributed to the considerable Cenozoic radiation of Neoisoptera, principally Termitidae, and their ecological dominance of tropical ecosystems101. Future research should, among other avenues, focus on fine comparisons of the alarm communication between termites and ants, or more generally speaking amongst all eusocial groups. These insects share common patterns of social organization, and ants have already been studied in respect to alarm communication in considerable detail23,102,103,104.
Material and methods
Biological material
Representative species from most major termite taxa, in addition to the wood roach Cryptocercus punctulatus, a member of the extant sister group to termites82,105,106, were used by using one colony for each in this study (Supplementary Data 1). Information from previous publications were reanalyzed and standardized to increase dataset coverage across species, and a newly acquired dataset of termite species was obtained from laboratory colonies and/or field colonies, following the protocols described below. All material was transported to Prague (Czech Republic) following legal procedures with the full array of permits from the country of origin. The combined dataset allowed for a comparative analysis of the evolution of alarm communication components in each termite taxon, which we linked with ecological or developmental traits. A detailed description of material origin, ecological and developmental traits, and methodological approaches is provided in Supplementary Data 1.
Behavioral experiments
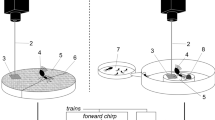
The experimental groups consisted of workers and soldiers maintained in their species-specific caste ratio (according to107; for details on caste ratio, see Supplementary Data 1) in a 85 mm Petri dish108. Only the species whose caste ratio is indicated in Supplementary Data 1 were studied. Tested stimuli consisted of (A) light—flash of three seconds (800 lux, 5500 – 6000 K color temperature), (B) air current—3 s of human breath through a fine straw to mimic a breach into a nest, (C) one crushed worker or soldier head spread on a piece of filter paper (CWH or CSH, respectively). (A) and (B) are hereafter called “direct disturbances”. All experimental groups were introduced in a Petri dish, and left for two hours undisturbed before being exposed to one of the disturbance stimuli. Stimuli and controls were replicated six times on independent groups by a single person, and recorded in full HD with Canon EOS 6D combined with EF 100 mm f/2.8 L Macro IS USM. Each video was recorded for a total of seven minutes, including one minute before the introduction of the stimulus, and six minutes after the stimulus was introduced. We then analyzed the short-term (1 min) and long-term (6 min) response of termites. The locomotion speed of two workers and two soldiers selected randomly (we used both soldiers in an experiment in species with a low soldier-to-worker ratio) were obtained from each replicate by using Mouse-Tracer software (ref. 36) and allowed us to learn about the presence or absence of an alarm pheromone in each species based on responses to CSH.
Chemical analysis
Substances that could be alarm pheromones were investigated in all focal species by chromatographic analysis. Cold-anesthetized termites were dissected using a stereomicroscope. Termite heads with the frontal gland from 2 to 20 individuals were placed into a 2-mL clear glass vial, crushed using a glass rod and the vial was closed with a PTFE/silicone septum cap. The headspace extraction of volatiles was carried out using an SPME fiber holder for manual sampling equipped with a fused silica fiber coated with 30 µm polydimethylsiloxane (Supelco, Bellefonte, USA). The holder needle was passed through the vial septum and the fiber was exposed for 10 min at room temperature. The analytes were desorbed at 220 °C in a split/splitless injector of a 5975B quadrupole mass spectrometer coupled to a 6890 N gas chromatograph (Agilent, Santa Clara, CA). The separation was achieved on a DB-5ms capillary column (30 m × 0.25 mm, a film thickness of 0.25 µm, Agilent) at a constant flow mode (1 ml/min) with helium as a carrier gas. The temperature program was: 40 °C (1 min), then 5 °C/min to 200 °C, then 15 °C/min to 320 °C (3 min). The temperatures of the transfer line, ion source, and quadrupole were 280 °C, 230 °C, and 150 °C, respectively. The compounds were ionized by 70 eV electrons.
Moreover, the tissue compounds were extracted with a small amount of hexane, i.e. the liquid extraction was achieved by adding 50–100 µl of hexane to freshly crushed termite heads. The extracts were analyzed on the same GC-MS instrument and the same column as above with slightly modified parameters. The injector held at 250 °C and operated with a split ratio of 1:20 injected 1 μl of the extract. The temperature program was as follow: 50 °C (1 min), then 15 °C/min to 200 °C, then 6 °C/min to 320 °C (3 min); total run time was 34 min. Data were recorded with a 4-min solvent delay. Detailed data on the identity of alarm pheromones originated from previous works of our team27,68,69.
Vibroacoustic experiments
The experiments were carried out on all the species indicated in Fig. 4 in an anechoic room, which provides low background noise. Prior to experiments, the experimental design of each 85 mm Petri dish (distance between floor and ceiling, coursing the floor, humidity, etc.) was optimized for each species. Videos were recorded with a Panasonic HDC-TM700 camera placed over the testing arenas, which allowed us to trace the origin of particular vibro-acoustic behaviors for each species and caste. The vibroacoustic recording system comprised high-sensitivity accelerometers (Brüel & Kjær type 4507 B 005) fixed on the bottom or on the lid of the Petri dish according to the species’ behavior. The accelerometric data were analyzed as overall alarm energy derived from the sum of tremulation and drumming after stimulation, normalized to the status before stimulation. The characteristics, nature, and frequency of beats composing bursts were analyzed until 50 observations were completed for each behavior. The entire records were analyzed in cases where less than 50 behavioral observations occurred. For the detailed procedure of data acquisition, see Fig. S6.
Reconstruction of ancestral states
We reconstructed the ancestral states of 33 characters (see Table S1) for 21 species using a phylogenetic tree of termites inferred from full mitochondrial genomes82,109. Alarm characters were selected from our observations and existing literature, ecological characters were selected according to our estimation of possible importance. The nature of the diet (column X31) was indicated according to existing literature110 and field observations. We used “Maximum parsimony” and “Maximum likelihood” methods, build-in Mesquite software (v3.6111), to estimate characters’ ancestral states. Because some analyses could not run with empty data, the characters with no values were removed prior the analyses.
Statistics
The behavioral experiments were evaluated using the Kruskal-Wallis test and post-hoc two-by-two permutation tests for independent samples (P values). The Bonferroni-Holm correction112 was applied for multiple comparisons among different conditions (H values). Accelerometric data were compared using t-tests for paired samples. All statistics were performed using StatXact (Cytel Studio, version 9.0.0, 2010) and SigmaPlot software (Systat Software Inc., version 11.0.0.77, 2007).
Character correlation analyses
To assess characters’ evolutionary correlations, we carried out a three-step approach. First, we performed statistical correlation using Chi-square test with p-value simulated by 500,000 iterations (chisq.test function in R 3.5.2.). Significant correlations were arbitrarily set to p < 0.01, to avoid random effects as much as possible. Secondly, we performed Pairwise Comparisons contrasting in state of two characters113 implemented in the software package Mesquite (v3.6111). In all cases, we always selected the Pairwise model with the highest “best tail” p-value for further scoring, and only scores above 0.8 were analyzed further. We scored every character pair in our dataset using the following equation:
in which
and
PW is the coefficient of taxa pairs in Pairwise comparison, based on difference between positive (Pos) and negative (Neg) pair sets in the pairwise model. nPW is the number of pairs in model. R is the coefficient of remaining taxa/nodes not included in any pair. R is expressed for all nodes, excluded from pairs connections defining clades with the same character states to avoid “pseudoreplication of lineage-specific factors”114. A clade is then defined as a separate node including terminals, if the sister clade differs in the given character state. nR+ is the number of nodes supporting PW, nR- is the number of nodes contradicting PW. nR is the total number of nodes not included in clades as defined above. Taxa containing unknown states in a particular character pair were omitted from the analysis. Lastly, we selected pairs of characters fitting both criteria and created the mirror trees (Mesquite software, v3.6111) to display the correlations.
MCA analysis of ecological traits effect on vibroacoustic communication
To determine the effects of environment and species ecology on means of vibratory communications, we performed Multiple Correspondence Analysis (MCA, libraries “FactoMineR” and “MissMDA” in R software, v3.5.2, https://www.r-project.org/). 14 characters (X21–X34, see Supplementary Data 1) were used as supplementary variables to 10 independent vibroacoustic alarm characters (X5–X14, see Supplementary Data 1).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
References
Stankowich, T., Haverkamp, P. J. & Caro, T. Ecological drivers of antipredator defenses in Carnivores. Evolution 68, 1415–1425 (2014).
Sugiura, S. Predators as drivers of insect defenses. Entomol. Sci. 23, 316–337 (2020).
Beauchamp, G. Animal Vigilance—Monitoring Predators And Competitors. p. 254 (Elsevier, 2015).
Hollén, L. I. & Radford, A. N. The development of alarm call behaviour in mammals and birds. Anim. Behav. 78, 791–800 (2009).
Gill, S. & Bierema, A. M.-K. On the meaning of alarm calls: a review of functional reference in avian alarm calling. Ethology 119, 449–461 (2013).
Blumstein, D. T. Rodent Societies: An Ecological & Evolutionary Perspective (eds. J. O. Wolff & P. W. Sherman) pp. 317–327. (University of Chicago Press, 2007).
Snowdon, C. T. Vervet monkey alarm calls: setting the historical context. Anim. Behav. Cogn. 7, 87–94 (2020).
Hamilton, W. D. The genetical evolution of social behaviour. J. Theor. Biol. 7, 1–52 (1964).
Smith, J. M. The evolution of alarm calls. Am. Nat. 99, 59–63 (1965).
Taylor, R. J., Balph, D. F. & Balph, M. H. The evolution of alarm calling: a cost-benefit analysis. Anim. Behav. 39, 860–868 (1990).
Hermann, H. R. Defensive Mechanisms in Social Insects. p. 259 (Praeger Publishers, 1984).
Stern, D. L. & Foster, W. A. The evolution of soldiers in aphids. Biol. Rev. 71, 27–79 (1996).
Smith, R. J. F. Alarm signals in fishes. Rev. Fish. Biol. Fish. 2, 33–63 (1992).
Klump, G. M. & Shalter, M. D. Acoustic behaviour of birds and mammals in the predator context; I. Factors affecting the structure of alarm signals. II. The functional significance and evolution of alarm signals. Z. Tierpsychol. 66, 189–226 (1984).
Caro, T. Antipredator Defenses in Birds and Mammals. p. 592 (University of Chicago Press, 2005).
Seyfarth, R. M., Cheney, D. L. & Marler, P. Monkey responses to three different alarm calls: Classification and semantic communication. Science 210, 801–803 (1980).
Wyatt, T. D. Pheromones and Animal Behaviour. p. 391 (Cambridge University Press, 2003).
Hill, P. S. M. Vibrational Communication in Animals. p. 272 (Harvard, Cambridge, 2008).
Hill, P. S. M. Biotremology: We fight for food. Curr. Biol. 29, R209–R212 (2019).
Cocroft, R. B. & Rodríguez, R. L. The behavioral ecology of insect vibrational communication. BioScience 55, 323–334 (2005).
Kettler, R. & Leuthold, R. H. Inter- and intraspecific alarm response in the termite Macrotermes subhyalinus (Rambur). Insectes Soc. 42, 145–156 (1995).
Hölldobler, B., Braun, U., Gronenberg, W., Kirchner, W. H. & Peeters, C. Trail communication in the ant Megaponera foetens (Fabr.) (Formicidae, Ponerinae). J. Insect Physiol. 40, 585–593 (1994).
Hunt, J. H. & Richard, F.-J. Intracolony vibroacoustic communication in social insects. Insectes Soc. 60, 403–417 (2013).
Howse, P. E. The perception of vibration by the subgenual organ in Zootermopsis angusticollis Emerson and Periplaneta americana L. Experientia 18, 457–458 (1962).
Golden, T. M. J. & Hill, P. S. M. The evolution of stridulatory communication in ants, revisited. Insectes Soc. 63, 309–319 (2016).
Šobotník, J., Hanus, R. & Roisin, Y. Agonistic behavior of the termite Prorhinotermes canalifrons (Isoptera: Rhinotermitidae). J. Insect Behav. 21, 521–534 (2008).
Delattre, O. et al. Complex alarm strategy in the most basal termite species. Behav. Ecol. Sociobiol. 69, 1945–1955 (2015).
Krausa, K., Hager, F. A., Kiatoko, N. & Kirchner, W. H. Vibrational signals of African stingless bees. Insectes Soc. 64, 415–424 (2017).
Rosengaus, R., Jordan, C., Lefebvre, M. & Traniello, J. F. A. Pathogen alarm behavior in a termite: a new form of communication in social insects. Naturwissenschaften 86, 544–548 (1999).
Evans, T. A. et al. Termites assess wood size by using vibration signals. Proc. Natl Acad. Sci. USA 102, 3732–3737 (2005).
Evans, T. A. et al. Termites eavesdrop to avoid competitors. Proc. R. Soc. B 276, 4035–4041 (2009).
Blum, M. S. Comprehensive Insect Physiology, Biochemistry and Pharmacology: Behavior, Vol. 9 (eds. G. A. Kerkut & L. I. Gilbert), pp. 193–224 (Pergamon Press, 1985).
Verheggen, F. J., Haubruge, E. & Mescher, M. C. Alarm pheromones—Chemical signaling in response to danger, Editor(s): Gerald Litwack. Vitam. Horm. 83, 215–239 (2010).
Šobotník, J., Jirošová, A. & Hanus, R. Chemical warfare in termites. J. Insect Physiol. 56, 1012–1021 (2010).
Eisner, T., Kriston, I. & Aneshansley, D. J. Defensive behavior of a termite (Nasutitermes exitiosus). Behav. Ecol. Sociobiol. 1, 83–125 (1976).
Šobotník, J. et al. (E,E)-α-Farnesene, an alarm pheromone of the termite Prorhinotermes canalifrons. J. Chem. Ecol. 34, 478–486 (2008).
Leonhardt, S. D., Menzel, F., Nehring, V. & Schmitt, T. Ecology and evolution of communication in social insects. Cell 164, 1277–1287 (2016).
Wilson, E. O. & Regnier, F. E. Jr The evolution of the alarm-defense system in the formicine ants. Am. Nat. 105, 279–289 (1971).
Bradshaw, J. W. S., Baker, R. & Howse, P. E. Multicomponent alarm pheromones of the weaver ant. Nature 258, 230–231 (1975).
Norman, V. C., Butterfield, T., Drijfhout, F., Tasman, K. & Hughes, W. O. Alarm pheromone composition and behavioral activity in fungus-growing ants. J. Chem. Ecol. 43, 225–235 (2017).
Prestwich, G. D. Defense Mechanisms of Termites. Annu. Rev. Entomol. 29, 201–232 (1984).
Bell, W. J., Roth, L. M. & Nalepa, C. A. Cockroaches: Ecology, Behavior, and Natural History. p. 247 (The Johns Hopkins University Press, 2007).
Evans, D. A., Baker, R., Briner, P. H. & McDowell, P. G. Defensive secretions of some African termites. Proceedings of the International Congress of the International Union for the Study of Social Insects (1977).
Anderson, M. The evolution of eusociality. Annu. Rev. Ecol. Syst. 15, 165–189 (1984).
Stern, D. L. A phylogenetic analysis of soldier evolution in the aphid family Hormaphididae. Proc. R. Soc. B 256, 203–209 (1994).
Bourke, A. F. Behavioural Ecology: An Evolutionary Approach, 4th ed. (eds. J. R. Krebs & N. B. Davies) pp. 203–227. (Blackwell Publishing, 1997).
Deligne, J., Quennedey, A. & Blum, M. S. Social Insects (ed. H. R. Hermann) p. 1–76 (Academic Press, 1981).
Bignell, D. E. The Mechanistic Benefits of Microbial Symbionts, Advances in Environmental Microbiology (ed. C. J. Hurst), p. 121–172. (Springer, 2016).
Bar-On, Y. M., Phillips, R. & Milo, R. The biomass distribution on Earth. Proc. Natl Acad. Sci. USA 115, 6506–6511 (2018).
Tuma, J., Eggleton, P. & Fayle, T. M. Ant-termite interactions: an important but under-explored ecological linkage. Biol. Rev. 95, 555–572 (2020).
Noirot, C. & Pasteels, J. M. Ontogenetic development and evolution of the worker caste in termites. Experientia 43, 851–860 (1987).
Prestwich, G. D., Bierl, B. A., Devilbiss, E. D. & Chaudhury, M. F. B. Soldier frontal glands of the termite Macrotermes subhyalinus: Morphology, chemical composition, and use in defense. J. Chem. Ecol. 3, 579–590 (1977).
Waller, D. A. & La Face, J. P. Unpalatability as a passive defense of Coptotermes formosanus Shiraki soldiers against ant predation. J. Appl. Entomol. 103, 148–153 (1987).
Quennedey, A. Defensive Mechanisms in Social Insects (ed. H. R. Hermann) p. 151–200. (1984)
Kuan, K.-C., Chiu, C. I., Shih, M.-C., Chi, K.-J. & Li, H.-F. Termite’s twisted mandible presents fast, powerful, and precise strikes. Sci. Rep. 10, 9462 (2020).
Šobotník, J. et al. Explosive backpacks in old termite workers. Science 337, 436 (2012).
Bourguignon, T. et al. Molecular mechanism of the two-component suicidal weapon of Neocapritermes taracua old workers. Mol. Biol. Evol. 33, 809–819 (2016).
Wilson, E. O. The Insect Societies (Harvard University Press, 1971).
Roisin, Y., Everaerts, C., Pasteels, J. M. & Bonnard, O. Caste-dependent reactions to soldier defensive secretion and chiral alarm/recruitment pheromone in Nasutitermes princeps. J. Chem. Ecol. 16, 2865–2875 (1990).
Connétable, S., Robert, A., Bouffault, F. & Bordereau, C. Vibratory alarm signals in two sympatric higher termite species: Pseudacanthotermes spiniger and P. militaris (Termitidae, Macrotermitinae). J. Insect Behav. 12, 329–342 (1999).
Lubin, Y. D. & Montgomery, G. G. Defenses of Nasutitermes termites (Isoptera, Termitidae) against Tamandua anteaters (Edentata, Myrmecophagidae). Biotropica 13, 66–76 (1981).
Hölldobler, B. & Wilson, E. O. The Ants (Harvard University Press, 1990).
Moore, B. P. Studies on the chemical composition and function of the cephalic gland secretion in Australian termites. J. Insect Physiol. 14, 33–39 (1968).
Maschwitz, U. & Mühlenberg, M. Chemische Gefahrenalarmierung bei einer Termite. Sci. Nat. 59, 516–516 (1972).
Vrkoč, J., Křeček, J. & Hrdý, I. Monoterpenic alarm pheromones in two Nasutitermes species. Acta Entomol. Bohemoslov. 75, 1–8 (1978).
Röhrig, A., Kirchner, W. H. & Leuthold, R. H. Vibrational alarm communication in the African fungus-growing termite genus Macrotermes (Isoptera, Termitidae). Insectes Soc. 46, 71–77 (1999).
Reinhard, J. & Clément, J.-L. Alarm reaction of European Reticulitermes termites to soldier head capsule volatiles (Isoptera, Rhinotermitidae). J. Insect Behav. 15, 95–107 (2002).
Cristaldo, P. F. et al. The nature of alarm communication in Constrictotermes cyphergaster (Blattodea: Termitoidea: Termitidae): the integration of chemical and vibroacoustic signals. Biol. Open 4, 1649–1659 (2015).
Delattre, O. et al. Chemical and vibratory signals used in alarm communication in the termite Reticulitermes flavipes (Rhinotermitidae). Insectes Soc. 66, 265–272 (2019).
Krishna, K., Grimaldi, D. A., Krishna, V. & Engel, M. S. Treatise on the Isoptera of the world. Bull. Am. Mus. Nat. Hist. 377, 1–2407 (2013).
Howse, P. E. On the significance of certain oscillatory movements of termites. Insectes Soc. 12, 335–345 (1965).
Stuart, A. M. Studies on the communication of alarm in the termite Zootermopsis nevadensis (Hagen), Isoptera. Physiol. Zool. 36, 85–96 (1963).
Šobotník, J., Bourguignon, T., Hanus, R., Weyda, F. & Roisin, Y. Structure and function of defensive glands in soldiers of Glossotermes oculatus (Isoptera: Serritermitidae). Biol. J. Linn. Soc. 99, 839–848 (2010).
Farine, J.-P. et al. The defensive secretion of Eurycotis floridana (Dictyoptera, Blattidae, Polyzosteriinae): chemical identification and evidence of an alarm function. Insect Biochem. Mol. Biol. 27, 577–586 (1997).
Farine, J.-P. et al. Defensive secretion of Therea petiveriana: Chemical identification and evidence of an alarm function. J. Chem. Ecol. 28, 1629–1640 (2002).
Brossut, R. Allomonal secretions in cockroaches. J. Chem. Ecol. 9, 143–158 (1983).
Noirot, C. Biology of Termites (eds. K. Krishna, F. M. Weesner), p. 89–123. (Academic Press, 1969).
Neoh, K.-B., Yeap, B.-K., Tsunoda, K., Yoshimura, T. & Lee, C.-Y. Do termites avoid carcasses? Behavioral responses depend on the nature of the carcasses. PLoS ONE 7, e36375 (2012).
Sun, Q., Hampton, J. D., Merchant, A., Haynes, K. F. & Zhou, X. Cooperative policing behaviour regulates reproductive division of labour in a termite. Proc. R. Soc. B 287, 20200780 (2020).
Lenz, M. Caste Differentiation in Social Insects (eds. J. A. L. Watson, B. M. Okot-Kotber, C. Noirot), p. 125–145. (Pergamon Press, 1985).
Stuart, A. M. The determination and regulation of the neotenic reproductive caste in the lower termites (Isoptera): with special reference to the genus Zootermopsis (Hagen). Sociobiology 4, 223–237 (1979).
Bourguignon, T. et al. The evolutionary history of termites as inferred from 66 mitochondrial genomes. Mol. Biol. Evol. 32, 406–421 (2015).
Buček, A. et al., Evolution of termite symbiosis informed by transcriptome-based phylogenies. Curr. Biol. 29 (2019).
Shellman-Reeve, J. S. The Evolution of Social Behaviour in Insects and Arachnids. (eds. B. J. Crespi, J. C. Choe), p. 52–93. (Cambridge University Press, 1997)
Seelinger, G. & Seelinger, U. On the social organisation, alarm and fighting in the primitive cockroach Cryptocercus punctulatus Scudder. Z. Tierpsychol. 61, 315–333 (1983).
Maistrello, L. & Sbrenna, G. Behavioural differences between male and female replacement reproductives in Kalotermes flavicollis (Isoptera, Kalotermitidae). Insectes Soc. 46, 186–191 (1999).
Maistrello, L. & Sbrenna, G. Frequency of some behavioural patterns in colonies of Kalotermes flavicollis (Isoptera Kalotermitidae): the importance of social interactions and vibratory movements as mechanisms for social integration. Ethol. Ecol. Evol. 8, 365–375 (1996).
Whitman, J. G. & Forschler, B. T. Observational notes on short-lived and infrequent behaviors displayed by Reticulitermes flavipes (Isoptera: Rhinotermitidae). Ann. Entomol. Soc. Am. 100, 763–771 (2007).
Ruhland, F., Moulin, M., Choppin, M., Meunier, J. & Lucas, C. Reproductives and eggs trigger worker vibration in a subterranean termite. Ecol. Evol. 10, 5892–5898 (2020).
Stuart, A. M. Preliminary studies on the significance of head-banging movements in termites with special reference to Zootermopsis angusticollis (Hagen) (Isoptera: Hodotermitidae). Sociobiology 14, 49–60 (1988).
Noirot, C. Biology of Termites (eds. K. Krishna, F. Weesner) Vol. 2, p. 73–125 (Academic Press, 1970).
Aguilera-Olivares, D., Palma-Onetto, V., Flores-Prado, L., Zapata, V. & Niemeyer, H. M. X-ray computed tomography reveals that intraspecific competition promotes soldier differentiation in a one-piece nesting termite. Entomol. Exp. Appl. 163, 26–34 (2017).
King, E. G. & Spink, W. T. Foraging galleries of the Formosan subterranean termite, Coptotermes formosanus, in Louisiana. Ann. Entomol. Soc. Am. 62, 536–542 (1969).
Valterová, I., Vrkoč, J., Lindström, M. & Norin, T. On the natural occurrence of (-)-3-carene, a component of termite defense secretions. Sci. Nat. 79, 416–417 (1992).
Nutting, W. L. Colonizing flights and associated activities of termites. I. The desert damp-wood termite Paraneotermes simplicicornis (Kalotermitidae). Psyche 73, 131–149 (1966).
Mizumoto, N. & Bourguignon, T. Modern termites inherited the potential of collective construction from their common ancestor. Ecol. Evol. 10, 6775–6784 (2020).
Sands, W. A. The soldierless termites of Africa. Bull. Br. Mus. Nat. Hist. (Suppl.) 18, 1–244 (1972).
Ahmad, M. The soldierless termite genera of the Oriental region, with a note on their phylogeny (Isoptera: Termitidae). Pak. J. Zool. 8, 105–123 (1976).
Miller, L. R. Invasitermes, a new genus of soldierless termites from northern Australia (Isoptera: Termitidae). J. Aust. Entomol. Soc. 23, 33–37 (1984).
Šobotník, J. et al. The frontal gland in workers of Neotropical soldierless termites. Naturwissenschaften 97, 495–503 (2010).
Chouvenc, T., Šobotník, J., Engel, M. S. & Bourguignon, T. Termite evolution: mutualistic associations, key innovations, and the rise of Termitidae. Cell. Mol. Life Sci. 78, 2749–2769 (2021).
Hermann, H. R. & Blum, M. S. Social Insects (ed. H. R. Hermann) Vol. 2, pp 77–197 (Academic Press, 1981).
Buschinger, A., Maschwitz, U. Defensive Mechanisms In Social Insects (ed. H. R. Hermann), p. 95–150 (Praeger, 1984).
Schönrogge, K., Barbero, F., Casacci, L. P., Settele, J. & Thomas, J. A. Acoustic communication within ant societies and its mimicry by mutualistic and socially parasitic myrmecophiles. Anim. Behav. 134, 249–256 (2017).
Lo, N. et al. Evidence from multiple gene sequences indicates that termites evolved from wood-feeding cockroaches. Curr. Biol. 10, 801–804 (2000).
Inward, D., Beccaloni, G. & Eggleton, P. Death of an order: a comprehensive molecular phylogenetic study confirms that termites are eusocial cockroaches. Biol. Lett. 3, 331–335 (2007).
Haverty, M. I. The proportion of soldiers in termite colonies: a list and a bibliography. Sociobiology 2, 199–216 (1977).
Miramontes, O. & DeSouza, O. The nonlinear dynamics of survival and social facilitation in termites. J. Theor. Biol. 181, 373–380 (1996).
Bourguignon, T. et al. Mitochondrial phylogenomics resolves the global spread of higher termites, ecosystem engineers of the tropics. Mol. Biol. Evol. 34, 589–597 (2017).
Donovan, S. E., Eggleton, P. & Bignell, D. E. Gut content analysis and a new feeding group classification of termites. Ecol. Entomol. 26, 356–366 (2001).
Maddison, W. P. & Maddison, D. R. Mesquite: A Modular System For Evolutionary Analysis http://mesquiteproject.org (2019).
Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65–70 (1979).
Maddison, W. P. Testing character correlation using pairwise comparisons on a phylogeny. J. Theor. Biol. 202, 195–204 (2000).
Maddison, W. P. & FitzJohn, R. G. The unsolved challenge to phylogenetic correlation tests for categorical characters. Syst. Biol. 64, 127–136 (2015).
Acknowledgements
We acknowledge our late friend, Philippe Cerdan, and also Régis Vigouroux and all Hydreco staff for their hospitality, and to František Jůna for recording behavior of Cryptocercus. We are grateful to Christine Nalepa (North Carolina State University, USA), who kindly provided us several family groups of Cryptocercus punctulatus used in this work, to Reinhard Leuthold who provided Hodotermes mossambicus, and to Alain Robert who provided Termes hospes and Hodotermopsis sjostedti. This study was supported by project CIGA No.2015430 (Czech University of Life Sciences Prague), by Faculty of Tropical AgriSciences (project IGA 20223112), and USDA National Institute of Food and Agriculture Hatch projects number FLA-FLT 005660. We also thank the anonymous reviewers and the editor for their helpful and constructive comments. Permits: Part of our material originated from colonies maintained in the lab since decades, when no specific permissions were required to import living material to Europe. Hodotermopsis sjostedti was collected by Alain Robert in Tam Dao forest (Vietnam) in 1990. Hodotermes mossambicus was collected by Reinhard Leuthold in Kenya in 1990. Neotermes cubanus was collected by Ivan Hrdý in Topes, de Collantes, Siera de Escambri (Cuba) in 1988. Glossotermes oculatus and Labiotermes labralis have been collected under permit approved by the French Ministry for the Ecological and Solidarity Transition (UID: ABSCH-CNA-FR-240495-2) (TREL1902817S/118). Termes hospes was collected by Alain Robert near Pointe-Noire in the Republic of the Congo. Cryptocercus punctulatus was generously provided by Christine Nalepa (North Carolina State University, USA). No new material of Constrictotermes cyphergaster was used in this study, but instead, we have re-analyzed available the data published in Cristaldo et al. 201568, where the details of legal procedures are provided. The same is true for Mastotermes darwiniensis and Reticulitermes flavipes, for which the details are available in Delattre et al. 201527 and Delattre et al. 201969, respectively.
Author information
Authors and Affiliations
Contributions
D.S.D., formulation of research strategy, supervisor of behavioral part, writing the M.S.; V.J., vibroacoustic recording and signal processing, data analysis, writing the MS; P.S., statistical and ecological modeling; O.D., behavioral tests with termites, compiling the literature sources; T.C., writing the MS, data analysis; O.B., behavioral tests with Cryptocercus, evaluation of behavior; J.C., chemical analyses; D.S., behavioral results evaluation; J.S., preparation of experiments, behavioral results evaluation; M.B. and O.J., evaluation of vibroacoustic data; M.S.E. and T.B., results presentation, text edits; J.Š., formulation of research strategy, funding, coordination of all activities, writing the MS.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Juliana Toledo Lima and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Karli Montague-Cardoso. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sillam-Dussès, D., Jandák, V., Stiblik, P. et al. Alarm communication predates eusociality in termites. Commun Biol 6, 83 (2023). https://doi.org/10.1038/s42003-023-04438-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-04438-5
This article is cited by
-
New comparative genomic evidence supporting the proteomic diversification role of A-to-I RNA editing in insects
Molecular Genetics and Genomics (2024)
-
Effect of soldiers on vibroacoustic alarm response in workers of subterranean termites (Blattodea: Rhinotermitidae)
Insectes Sociaux (2024)
-
Differentiation of workers into soldiers is associated with a size reduction of higher-order brain centers in the neotropical termite Procornitermes araujoi
Scientific Reports (2023)
-
The Trail-Following Communication in Stylotermes faveolus and S. halumicus (Blattodea, Isoptera, Stylotermitidae)
Journal of Chemical Ecology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.