Abstract
Endochondral ossification is regulated by transcription factors that include SRY-box transcription factor 9, runt-related protein 2 (Runx2), and Osterix. However, the sequential and harmonious regulation of the multiple steps of endochondral ossification is unclear. This study identified zinc finger homeodomain 4 (Zfhx4) as a crucial transcriptional partner of Osterix. We found that Zfhx4 was highly expressed in cartilage and that Zfhx4 deficient mice had reduced expression of matrix metallopeptidase 13 and inhibited calcification of cartilage matrices. These phenotypes were very similar to impaired chondrogenesis in Osterix deficient mice. Coimmunoprecipitation and immunofluorescence indicated a physical interaction between Zfhx4 and Osterix. Notably, Zfhx4 and Osterix double mutant mice showed more severe phenotype than Zfhx4 deficient mice. Additionally, Zfhx4 interacted with Runx2 that functions upstream of Osterix. Our findings suggest that Zfhx4 coordinates the transcriptional network of Osterix and, consequently, endochondral ossification.
Similar content being viewed by others
Introduction
Vertebrate skeletal elements are formed through two distinct processes: intramembranous and endochondral ossifications1,2. Intramembranous ossification is the process by which mesenchymal cells differentiate into osteoblasts and is consequently responsible for the formation of the clavicle and most of the craniofacial skeleton3. Conversely, endochondral ossification involves numerous steps of chondrocyte differentiation followed by replacement of cartilage tissue with bone tissue, which gives rise to most bones that include vertebrae, ribs, and long bones1,4. The first step of endochondral ossification is mesenchymal condensation after which cells differentiate into resting and proliferating chondrocytes that produce components of the chondrogenic extracellular matrix including collagen type 2 alpha 1 chain (Col2a1), collagen type 11 alpha 2 chain (Col11a2), and aggrecan (Acan)5,6. The chondrocytes then differentiate into hypertrophic chondrocytes that produce collagen type 10 alpha 1 chain (Col10a1), Indian hedgehog protein (Ihh), and matrix metalloproteinase 13 (Mmp13)7,8,9,10. Subsequently, hypertrophic chondrocytes undergo apoptosis, cartilage matrices are calcified, vascular vessels invade the cartilage tissue and, finally, cartilage tissues are replaced by bone tissue11. These unique and complex steps are regulated by various critical transcription factors and coactivators10.
The transcription factor SRY-box transcription factor 9 (Sox9), is essential for the early stage of chondrogenesis, in which mesenchymal condensation and chondrocyte differentiation occur12. Mutations in the SOX9 gene in humans cause campomelic dysplasia (OMIM 114290) characterized by severe chondrodysplasia13,14. Similarly, mutations around the SOX9 gene cause Pierre Robin sequence (OMIM 261800) that involves a cleft palate15. The expression of transcriptional factors Sox5 and Sox6, both of which are crucial for early chondrogenesis, is induced by Sox916,17,18. A critical role of Sox9 in chondrogenesis has also been demonstrated by a study in which chondrogenesis was severely impaired in chondrocyte-specific Sox9 conditional knockout (KO) mice19. Moreover, Sox9 directly regulates the expression of early-stage chondrogenic genes that include Col2a1, Col11a2, and Acan6,16,20.
Conversely, runt-related protein (Runx) 2, Runx3, and Osterix are crucial for the late stages of chondrogenesis. Chondrocyte hypertrophy is severely impaired in Runx2 KO mice, whereas Runx2 and Runx3 double KO mice exhibit a complete lack of hypertrophic chondrocytes21. Furthermore, Runx2 plays a critical role in the regulation of Col10a1, Ihh, and vascular endothelial growth factor (Vegf)21,22,23. Osterix (also known as Sp7) functions as a downstream transcriptional partner of Runx2 during bone and cartilage development10. Chondrocyte-specific Osterix conditional KO mice show a lack of calcification in cartilage matrices and matrix vesicles as well as loss of Mmp13 expression10. Thus, the Sox9-Runx2-Osterix axis is responsible for spatiotemporal mediation of endochondral ossification.
Transcriptional partners of Sox9 and Runx2 have been identified, which interact with the proteins during endochondral ossification. Peroxisome proliferator-activated receptor co-activator 1α forms a complex with Sox924 similarly to p300/CREB-binding protein25, protein 54 kDa nuclear RNA-binding protein (p54nrb)26, AT-rich interactive domain (Arid) 5a27, Arid5b28, zinc finger protein 21929, and WW domain-containing E3 ubiquitin protein ligase 2 (Wwp2)30,31. Core-binding factor beta (Cbfβ) forms a heterodimer with Runx221,32,33, which also interacts with CCAAT/enhancer binding protein β34.
Considering the sequential and harmonious regulation of endochondral ossification achieved through this transcriptional network, it is possible that transcription factors that have not been identified may be involved in the multiple steps of cartilage development.
The present study aimed to identify novel transcription factors involved in the regulation of endochondral ossification and to elucidate the functional roles of these factors. We isolated zinc finger homeobox 4 (Zfhx4) from mouse limb buds and identified it as a highly expressed transcription factor in chondrogenic tissues. We demonstrated that Zfhx4 is required for endochondral ossification in conjunction with Osterix. We also found that Zfhx4 physically interacts with Runx2. Because Zfhx4 is a large transcription factor with four homeodomains and 22 zinc finger domains, Zfhx4 might function in the transcriptional platform and interact with several transcription factors and transcriptional regulators. Our findings provide insights into the role of Zfhx4 in the regulation of endochondral ossification.
Results
Identification of Zfhx4 as a transcription factor highly expressed in chondrocytes
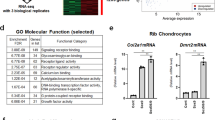
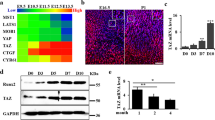
Microarray analysis revealed high expression levels of p54nrb, Arid5b, Bbf2h7, Cbfβ, ATF4, Sox9, Sox5, Sox6, Wwp2, Runx2, Dlx5, Dlx6, Msx2, and Osterix in mouse limb bud cells, all of which play important roles in endochondral ossification (Fig. 1a). Furthermore, Zfhx4 was highly expressed in limb bud cells (Fig. 1a). We focused on Zfhx4 for further analyses because of its putative role in 8q21.11 microdeletion syndrome (OMIM 614230) characterized by skeletal abnormalities35. Figure 1b shows the results of reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR) analyses of newborn mouse tissues, which showed that Zfhx4 was highly expressed in several skeletal tissues that included ribs, limbs, and the calvaria. Consistent with the RT-qPCR data, expression of Zfhx4 was detected in limb bud cells by whole mount in situ and in situ hybridization analyses (Supplementary Fig. 1a, b). Additionally, in situ hybridization showed high expression of Zfhx4 in the growth plate of embryonic day (E) 16.5 mouse femurs (Fig. 1c).
a List of highly expressed transcription factors in limb buds determined by microarray analysis. b Expression of Zfhx4 in tissues of newborn mice. Total RNA was isolated from the indicated tissues and analyzed by RT-qPCR. Data are shown as the mean ± s.d. (n = 3). c Expression of Zfhx4 in the growth plate of mice. Hematoxylin and eosin staining (HE) and in situ hybridization (ISH) were carried out on growth plate chondrocytes of femurs from E16.5 mice. Scale bar = 500 μm. d Macroscopic views of the palate of P0 wild-type (WT) and Zfhx4−/− littermates. Cleft palates were observed in Zfhx4−/− mice. Scale bar = 1 mm. e Skeletal preparations of whole embryos, ribs, mandibles, skulls, forelimbs, and hindlimbs of E15.5 WT and Zfhx4−/− littermates stained with Alcian blue and alizarin red. Scale bar = 1 mm.
Presence of skeletal abnormalities and cleft palate in Zfhx4-deficient mice
To generate Zfhx4-deficient mice, we first generated Zfhx4 floxed mice (Supplementary Fig. 1c) and confirmed germline transmission by Southern blot and PCR analyses (Supplementary Fig. 1d). The generation of Zfhx4 heterozygous-deficient mice by further crossing was confirmed by PCR.
Zfhx4 homozygous deficient (Zfhx4−/−) mice died within 1 day after birth, exhibited a cleft palate (Fig. 1d), and became cyanotic with gasping respirations and air-distended stomachs (Supplementary Fig. 2). Moreover, Zfhx4−/− mice exhibited skull malformations and shorter humeri and femurs than wild-type littermates and suffered loss of the condylar process and hypoplasia of the tracheal–bronchial ring at P0 (Supplementary Figs. 3a and 4). Zfhx4−/− embryos showed skeletal hypoplasia of the skull, mandible, humerus, femur, and ribs at E15.5 and E16.5 (Fig. 1e and Supplementary Fig. 3b). Notably, delayed ossification of the humerus and femur of Zfhx4−/− mice was observed at E15.5 and E16.5 (Fig. 1e and Supplementary Fig. 3b).
Impaired endochondral ossification in Zfhx4-deficient mice
Histological analysis revealed that E15.5 Zfhx4−/− mice had very few hypertrophic chondrocytes, although wild-type littermates exhibited appropriate hypertrophic chondrocyte zones (Fig. 2a). Additionally, E16.5 Zfhx−/− mice showed impairment of calcification in cartilage matrices (Fig. 2b). In situ hybridization revealed normal expression of Sox9 and Col2a1 in E15.5 Zfhx4−/− mice. However, such expression was not separated at the diaphysis (Fig. 2c). Ihh, Ppr, Runx2, and Osterix were expressed, but not separated at the diaphysis in Zfhx4−/− mice compared with wild-type littermates (Fig. 2c). Most strikingly, no expression of Col10a1 or Mmp13 was detected in Zfhx4−/− mice (Fig. 2c). Immunofluorescence staining and RT-qPCR analyses also showed very little expression of Mmp13 in Zfhx−/– mice (Fig. 2d, e).
a Hemolysin and eosin (HE)-stained femurs of E15.5 wild-type (WT) and Zfhx4−/− littermates. Scale bar = 500 μm. b Von Kossa-stained femurs of E16.5 WT and Zfhx4−/− littermates. Scale bar = 200 μm. c Expression of chondrogenic gene markers in Zfhx4−/− mice. Paraffin-embedded sections of femurs from E15.5 WT and Zfhx4−/− littermates were examined by in situ hybridization using anti-sense probes against Sox9, Col2a1, Ihh, Ppr, Runx2, Osterix, Col10a1, and Mmp13. Scale bar = 500 μm. d Immunofluorescence analysis of femurs from E15.5 WT and Zfhx4−/− littermates using anti-Col2, anti-Col10, and anti-Mmp13 antibodies. Scale bar = 500 μm. e mRNA expression levels of chondrocyte marker genes. mRNA isolated from humeri of E15.5 WT and Zfhx4−/− littermates was analyzed by RT-qPCR. Data are presented as the mean ± s.d. (WT: n = 5; KO: n = 3).
Interaction of Zfhx4 with Osterix during endochondral ossification
Because the skeletal phenotype of Zfhx4−/− mice resembled that of Osterix KO mice with reduced Mmp13 expression and calcification of cartilage matrices10, we hypothesized that Zfhx4 interacted with Osterix during endochondral ossification. Coimmunoprecipitation and immunofluorescence analyses demonstrated that Zfhx4 physically associated with Osterix and that the proteins were colocalized in the nucleus (Fig. 3a, b). These results clearly demonstrated an association of Zfhx4 with Osterix. To understand the importance of this finding in terms of endochondral ossification, we generated double mutant mice of Zfhx4 and Osterix. Because Osterix KO mice exhibit a complete lack of Mmp13 expression and calcification of cartilage matrices10, we attempted to generate Zfhx4−/−;Osterix+/− mutant mice and compared the phenotype with Zfhx4−/− mice. Double mutant Zfhx4+/−;Osterix+/− mice appeared to be similar to wild-type mice (Fig. 3c–f, Supplementary Fig. 5a). Conversely, Zfhx4−/−;Osterix+/− mutant mice had less calcification of the humerus and femur at E16.5 than Zfhx4−/− littermates (Fig. 3c). Histological analyses indicated that hypertrophy and calcification of the femur were more severely impaired in Zfhx4−/−;Osterix+/− mice at E16.5 than in Zfhx4−/− littermates (Fig. 3d, e, Supplementary Fig. 6). Moreover, Mmp13 expression was diminished in Zfhx4−/−;Osterix+/− mice even at E16.5 when Zfhx4−/− littermates exhibited marginal expression of Mmp13 (Fig. 3f). Zfhx4−/−;Osterix+/− mice also showed more severe impairment at E15.5 than Zfhx4−/− littermates (Supplementary Fig. 5b, c).
a Physical interaction of Zfhx4 with Osterix. Coimmunoprecipitation analysis of lysates of 293FT cells transfected with Flag-Zfhx4, 6xMyc-Osterix, or both is shown. Closed arrows: 6xMyc-Osterix, Open arrow: Flag-Zfhx4 (IP immunoprecipitation, WB western blotting). b Colocalization of Flag-Zfhx4 (red) with Venus-Osterix(green) in SW1353 cells. Scale bar = 5 μm. c Skeletal preparations of whole bodies, forelimbs, and hindlimbs of E16.5 wild-type (WT), Zfhx4+/−;Osterix+/−, Zfhx4−/−, and Zfhx4−/−;Osterix+/− littermates stained with Alcian blue and alizarin red. Scale bar = 1 mm. Right panels show higher magnification images of the forelimbs and hindlimbs shown in the left panel. d–f Paraffin-embedded sections of E16.5 WT, Osterix+/−, Zfhx4+/−;Osterix+/−, Zfhx4−/−, and Zfhx4−/−;Osterix+/− littermates subjected to hematoxylin and eosin (HE) staining (d), von Kossa staining (e), and immunofluorescence staining with an anti-Mmp13 antibody (f). Scale bar = 500 μm.
It has been shown that Mmp13 expression is regulated by a complex formed by Runx2 and Osterix10. Supplementary Figure 7a shows the results of coimmunoprecipitation of Zfhx4 and Runx2, which revealed that the two proteins interacted physically and were localized to the nucleus (Supplementary Fig. 7b). Consistent the notion that Runx2 is an upstream transcription factor of Osterix10,36, Osterix was not required for the interaction of Zfhx4 with Runx2 (Supplementary Fig. 7c, d). We next examined the role of Zfhx4 in regulation of the Mmp13 gene promoter. A luciferase reporter assay indicated that Zfhx4 upregulated Mmp13 gene promoter activity and increased transcriptional activities of Osterix and Runx2 on their promoters (Supplementary Fig. 8a). Consistently, chromatin immunoprecipitation analyses revealed binding of Zfhx4, Osterix, and Runx2 to Mmp13 gene promoter regions (Supplementary Fig. 8b). Because Runx2 had a different binding ability to the Mmp13 gene promoter from Zfhx4 and Osterix, Zfhx4 may sequentially interact with Runx2 and Osterix.
Role of Zfhx4 in palate development
Because Zfhx4−/− mice showed a complete cleft palate at 100% penetrance (Fig. 1d, Supplementary Fig. 9a), we examined expression of Zfhx4 in the palatal shelf. In situ hybridization analysis clearly indicated that Zfhx4 was expressed in the palatal shelf of E13.5 mice (Fig. 4a). Examination of palate development of wild-type and Zfhx4−/− littermates at E13.5, E14.5, and E16.5 (Fig. 4b and Supplementary Fig. 9b–d) revealed that the growth and elongation of palatal shelves were normal in both Zfhx4−/− and wild-type E13.5 mice. Palatal shelves were fused in E14.5 wild-type mice, whereas palatal shelves of Zfhx4−/− mice were not elevated and had failed to fuse by E14.5 (Fig. 4b and Supplementary Fig. 9c). Palatal shelves of E16.5 Zfhx4−/− mice were degraded (Fig. 4b and Supplementary Fig. 9d). Consistent with the results indicating that growth and elongation of palatal shelves were normal in Zfhx4−/− mice, immunofluorescence with anti-Pcna and -Ki67 antibodies indicated normal cell proliferation in the palatal shelf of Zfhx4−/− mice (Fig. 4c, d). Additionally, there was no detectable difference in the number of apoptotic cells in the palatal shelf between Zfhx4−/− and wild-type littermate mice (Supplementary Fig. 10), which suggested that apoptosis was not involved in the etiology of cleft palate in Zfhx4−/− mice. To further examine the role of Zfhx4 in palatal development, we performed organ culture experiments using maxilla of E14.2 Zfhx4−/− mice. Unexpectedly, palatal selves of Zfhx4−/− mice were elevated and fused as well as wild-type littermates (Supplementary Fig. 11). These results suggested that Zfhx4 was unnecessary to elevate the palatal shelf itself.
a Zfhx4 expression in the palatal shelf of E13.5 mice determined by in situ hybridization. Scale bar = 200 μm. b Coronal sections of crania of E13.5, E14.5, and E16.5 wild-type (WT) and Zfhx4−/− littermates stained with hematoxylin and eosin (HE). Scale bar = 500 μm. c Fluorescence microscopy images of coronal sections of crania of E13.5 WT and Zfhx4−/− littermates subjected to immunofluorescence analysis using an anti-Pcna antibody. Scale bar = 100 μm. d Coronal sections of crania of E13.5 WT and Zfhx4−/− littermates subjected to immunofluorescence analysis using an anti-Ki67 antibody. Scale bar = 100 μm. e Expression levels of genes associated with palatal development in the palatal shelves of E13 WT and Zfhx4−/− littermates as determined by RT-qPCR using TaqMan probes. Data represent the mean ± s.d. (WT: n = 3; KO: n = 3). f Coronal sections of crania of E13.5 WT and Zfhx4−/− littermates were examined by in situ hybridization using anti-sense probes against Osr1, Osr2, and Pax9. Scale bar = 200 μm.
Palatal development involves multiple steps, which include palatal shelf outgrowth, elevation, adhesion, and fusion, and palatal bone formation. Odd-skipped-related (Osr) 137,38, Osr237,38, fibroblast growth factor 10 (Fgf10)39, msh homeobox 1 (Msx1)40,41, and Paired box 9 (Pax9)42,43 play essential roles in palatal development. Cranial neural crest-specific Sox9 conditional KO mice44 and Runx2 KO mice45 exhibit cleft palates. In the present study, we found no significant difference between wild-type and Zfhx4−/− littermates in terms of the expression of Osr1, Osr2, Fgf10, Msx1, or Pax9 in palatal shelves (Fig. 4e, f and Supplementary Fig. 12). The expression of Runx2 and Osterix also remained unchanged in Zfhx4−/− compared with wild-type littermates (Fig. 4e). Therefore, these results suggested that expression of these genes was not regulated and unknown Zfhx4 target genes might be involved in palatal development. Identification of target genes of Zfhx4 in the palatal shelf may contribute to understanding the molecular basis of palatal development, which involves Zfhx4.
Discussion
Cell differentiation, proliferation, and death are strictly regulated by transcriptional machinery complexes that comprise specific transcriptional factors and transcriptional coregulators. Although transcriptional regulation during endochondral ossification has been extensively investigated1, the details of the sequential and harmonious mechanism have not yet been elucidated. To address this, we attempted to identify transcription factors that regulate the transcriptional network system in endochondral ossification. In this study, we identified Zfhx4 as a transcriptional factor that is highly expressed in cartilage and limb buds. Notably, we found that Zfhx4 functioned as a transcriptional partner of Osterix. Coimmunoprecipitation and immunofluorescence analyses indicated a physical interaction between Zfhx4 and Osterix. Furthermore, genetic experiments revealed that Zfhx4 and Osterix interacted functionally in the late stage of endochondral ossification. Our data also indicated that Mmp13 expression was regulated by Zfhx4. Additionally, luciferase reporter and chromatin immunoprecipitation analyses indicated regulation of the Mmp13 gene promoter by Zfhx4. Because Osterix is critical for the regulation of Mmp13 expression in chondrocytes10, our results support the importance of the association between Zfhx4 and Osterix in endochondral ossification.
Col10a1 expression was diminished in Zfhx4−/− mice as determined by in situ hybridization experiments (Fig. 2c). Conversely, we observed similar expression level of Col10a1 and Col10 between wild-type and Zfhx4−/− littermates (Fig. 2d and e), although the delayed expression pattern of Col10 was consistent with the in situ hybridization experiments. We assume that these difference resulted from slightly different time points of these experiments. Therefore, we believe that Col10a1 is not a critical transcriptional target for Zfhx4.
Because of the structure of Zfhx4, it is possible that Zfhx4 associates with numerous transcriptional regulators that function in the platform of the transcriptional network during endochondral ossification. In addition to Osterix, we found that Zfhx4 physically interacted with Runx2, a critical transcription factor in endochondral ossification46. Furthermore, Runx2 functions upstream of Osterix by controlling Mmp13 expression through its interaction with Osterix10. Thus, our study suggests that Zfhx4 facilitates the Runx2-Osterix axis, which results in coordination of the sequential steps of the transcriptional network that underlies endochondral ossification.
We found that endochondral ossification was impaired in the proximal limbs of Zfhx4-deficient mice, which included the scapula, humerus, and femur. The anterior–posterior patterning of the proximal limb is regulated by homeobox (Hox) 9 and 10 paralogous groups47,48,49,50 as well as short stature homeobox 2 (Shox2)51. These genes are expressed in a spatiotemporally restricted pattern in domains along the axes of limb buds and provide anterior–posterior positional information for proximal limb patterning. Hoxa9/Hoxd9 double mutant mice have shortened humeri and loss of deltoid crests50, and triple mutant mice (Hoxa10/Hoxc10/Hoxd10) show short humeri, loss of deltoid processes, and remarkably shortened femurs49. Mutation of Shox2 causes marked shortening of the humerus and femur, as well as loss of the deltoid crest and ossification of the femur52. Double mutation of HoxD and Shox2 (HoxD−/−;Shox2+/−) causes the phenotype of shortened humeri and lack of deltoid crests, whereas HoxD+/+;Shox2+/− mutants show intact proximal limbs51. Considering the morphological phenotypes of Hox9, Hox10, and Shox2 mutant mice, Zfhx4 may be involved in the patterning processes of proximal limbs, possibly in association with Hox9, Hox10, and Shox2. Clinically, shortening of limbs is classified into four types: rhizomelic (dysplasia of proximal parts of the limbs), mesomelic (dysplasia of middle parts of the limbs), acromelic (dysplasia of distal parts of the limbs), and micromelic (generalized shortening of entire limbs)53. On the basis of the skeletal phenotype of Zfhx4-deficient mice, this mutation induces rhizomelic type skeletal dysplasia. Rhizomelic type skeletal dysplasia, achondroplasia, rhizomelic chondrodysplasia punctata, and spondyloepiphyseal dysplasia congenita are well characterized and several genes have been identified to be responsible for these diseases53. Achondroplasia (OMIM100800) is caused by heterozygous mutations in the gene that encodes fibroblast growth factor receptor 354, rhizomelic chondrodysplasia punctata type1 (RCDP1) (OMIM 215100) is caused by homozygous or compound heterozygous mutations in the gene that encodes peroxisomal biogenesis factor 7 (PEX7)55, and spondyloepiphyseal dysplasia congenital (OMIM183900) is caused by heterozygous mutations in COL2A156. However, the causative genes and pathological mechanisms of some rhizomelic type skeletal dysplasias, which include cleidorhizomelic syndrome, Patterson-Lowry rhizomelic dysplasia, and multiple epiphyseal dysplasia with Ribbing type, are yet to be elucidated. The Zfhx4 mutation described in the present study may be a good model to evaluate the pathogenesis of rhizomelic type skeletal dysplasia and such assessment might provide insights into the pathogenesis of rhizomelic dysplasia.
Our finding that Zfhx4 is highly expressed in calvariae suggests that Zfhx4 plays roles in both membranous and endochondral ossifications. However, Zfhx4-deficient mice showed no remarkable phenotype in terms of membranous ossification. We therefore assume that Zfhx4 has a partially redundant role and that its function might be compensated by other genes. A potential candidate gene is zinc finger homeobox 3 (Zfhx3; also known as ATBF1) that belongs to the Zfhx family and has the highest homology with Zfhx4 of all family members. The Zfhx3 protein is 406 kDa and contains four homeodomains and 23 zinc finger domains57. Supporting our theory, P10 Zfhx3 heterozygous mice are much smaller in terms of body size and weight than wild-type littermates58. This is presumably due to impaired skeletal development. Although dissection of Zfhx3/Zfhx4 double mutant mice may provide insights into the potential roles of Zfhx family proteins in skeletal development, this was beyond the scope of the present study. We intend to carry this out as the next step of this study.
We unexpectedly identified cleft palate at the elevation stage in Zfhx4-deficient mice. Notably, Zfhx4 was specifically expressed in palatal shelves, but not in the tongue. However, organ culture experiments showed that elevation and fusion abilities of palatal shelves from Zfhx4−/− mice and wild-type littermates were intact. An explanation for this discrepancy between in vivo and ex vivo experiments is that Zfhx4 is required to remove the tongue from the middle of developing palatal shelved to create the space for palatal shelves to elevate and fuse. Considering specific expression of Zfhx4 in palatal shelves, it is likely that Zfhx4 regulates the expression of genes involved in palatal development. However, the expression levels of well-known palatal marker genes appeared normal in Zfhx4-deficient mice. Further investigation is required to identify Zfhx4 target genes in palatal shelves. The Zfhx4 gene has been proposed to be a candidate gene responsible for 8q21.11 microdeletion syndrome35 and a report has shown this disease manifesting as a cleft palate in eight unrelated individuals. Thus, Zfhx4-deficient mice might be a suitable animal model to investigate the molecular mechanisms of palatal development and the pathogenesis of 8q21.11 microdeletion syndrome.
In conclusion, our results demonstrate that Zfhx4 is a transcriptional partner of Osterix and regulates the late stage of endochondral ossification. Our findings contribute to improving our understanding of the sequential and harmonious regulation of endochondral ossification.
Methods
Cell culture
Anterior and posterior limb buds were dissected from E12.5 Institute of Cancer Research (ICR) mouse embryos (SLC, Shizuoka, Japan) and digested by incubation in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma–Aldrich, St. Louis, MO, USA) with 0.1% collagenase type II (Sigma–Aldrich) and 0.1% trypsin (Sigma–Aldrich) at 37 °C29. Cells were dissociated by pipetting and then centrifuged at 300 × g for 5 min. The supernatant was removed and the cell pellet was resuspended with α-minimum essential medium (Sigma–Aldrich) with 10% fetal bovine serum (FBS; JRH Bioscience, Lenexa, KA, USA) to a final density of 2 × 107 cells/ml. Cells were incubated at 37 °C in a humidified atmosphere with 5% CO2. 293FT and SW1353 cell lines were purchased from Thermo Fisher Scientific (Waltham, MA, USA) and the American Type Culture Collection (Manassas, VA, USA), respectively. Cells were cultured in DMEM with 10% FBS at 37 °C in a humidified atmosphere with 5% CO2.
Animal experiments
All animal experiments were approved by the Osaka University Graduate School of Dentistry animal care committee and the Institutional Animal Care and Use Committee (IACUC) of RIKEN Kobe Branch. Animal experiments with E12, E12.5, E13.5, E14.5, E15.5, and E16.5 embryos, and newborn littermates were performed by following protocols approved by the Osaka University Graduate School of Dentistry animal care committee. CAG-Cre transgenic mice (RBRC01828) were provided by the RIKEN BioResource Research Center (Ibaraki, Japan).
Zfhx4 mutant mice were designed with floxed exons 7 and 8 (Supplementary Fig. S1c). Deletion of exons 7 and 8 caused a nonsense frame-shift mutation in most functional domains of the Zfhx4 protein, including all homeobox domains, 13 zinc finger domains and a region coding nuclear localization signal. To generate Zfhx4 floxed mice (accession number CDB0954K, http://www2.clst.riken.jp/arg/micelist.html), TT2 embryonic stem (ES) cells were transfected with a targeting vector that contained a neomycin resistance gene (Supplementary Fig. S1c) by electroporation59. Homologous recombination was confirmed by Southern blot analysis. The ES cells were injected into eight-cell-stage ICR embryos and Zfhx4 floxed chimera mice were obtained. The Zfhx4 floxed chimeras were mated with C57BL/6 mice and then germline transmission of the mutant allele was achieved as confirmed by Southern blotting and genomic PCR analyses. The 590 bp probe (Supplementary information) located in exon 10 of the Zfhx4 gene (Supplementary Fig. 1c) was used for Southern blotting analyses of EcoRI-digested genomic DNA from mouse tails. The sequences of primers used for genomic PCR were as follows: 5′-GCCAAAGGCTGACTCAAAAC-3′ and 5′-GGGTCCCCACTGTGATTTCT-3′. To generate Zfhx4 global knockout mice, heterozygous Zfhx4 floxed mice were mated with CAG-Cre transgenic mice. Deletion of the floxed allele was confirmed by genomic PCR. Deletion of the Cre transgene was achieved by mating Zfhx4 heterozygous-deficient mice with C57BL/6 mice as confirmed by genomic PCR. Zfhx4-deficient mice were produced by interbreeding of Zfhx4 heterozygous mice. To determine mouse genotypes, the following primers were used in PCR: 5′-TCACTGTGCATGAGGCAAAAC-3′ and 5′-GGGTCCCCACTGTGATTTCTA -3′. Osterix KO mice were used as described previously10.
Microarray analysis
We analyzed microarray data deposited in the Gene Expression Omnibus (Accession No: GSE126945). Briefly, total RNA was isolated from limb buds of E12.5 ICR mice using an Ambion® WT Expression Kit (Applied Biosystems, Branchburg, NJ, USA). Single-stranded DNA was synthesized from total RNA and subsequently hybridized to a GeneChip®Mouse Gene 1.0 ST Array (Affymetrix, Santa Clara, CA, USA). After 16 h of hybridization, the array was washed, scanned, and finally quantified by an Affymetrix Expression Console (Affymetrix)10.
Reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells using a NucleoSpin RNA Plus Kit (Macherey-Nagel, Duren, Germany). Total RNA was denatured by incubation at 65 °C for 5 min, after which cDNA was synthesized using ReverTra Ace Quantitative PCR RT Master Mix with gDNA Remover (Toyobo, Osaka, Japan)27. Real-time RT-qPCR amplification was performed using the TaqMan PCR protocol and Step-One plus real-time PCR system (Applied Biosystems, Carlsbad, CA, USA). TaqMan probes used for amplification are listed in Table 1. The expression level of mRNA was normalized to β-actin mRNA expression60.
Histological and immunohistochemical analyses
Samples were fixed in 4% paraformaldehyde/phosphate-buffered saline (PFA/PBS) overnight at 4 °C, embedded in paraffin, and cut into 7 μm-thick sections. The sections were deparaffinized, rehydrated, and then stained with Mayer’s hematoxylin and eosin (H&E). For antigen retrieval, deparaffinized sections were incubated with 5% hyaluronidase in PBS for 30 min at 37 °C for Col2, Col10, and Mmp13 immunostaining. After blocking with 5% bovine serum albumin (BSA), sections were incubated with anti-Col2 (1:1000, #7050; Woodinville Chondrex, WA, USA), anti-Col10 (1:500, LSL, Tokyo, Japan), anti-Mmp13 (1:200, ab39012; Abcam, Cambridge, UK), anti-Pcna (1:1000, #13110; Cell Signaling Technology, Danvers, MA, USA), or anti-Ki67 (1:200, ab15580; Abcam) antibodies at 4 °C for 16 h, washed thrice with PBS, and then incubated with Alexa Fluor 555-conjugated anti-rabbit immunoglobulin G (IgG) (1:500, A21429; Thermo Fisher Scientific) or anti-mouse IgG (1:500, A21424; Thermo Fisher Scientific)28,61. Sample immunoreactivity was analyzed under a fluorescence microscope (Leica DM4 B; Leica Microsystems, Wetzlar, Germany).
Skeletal preparation
The skin and internal organs of E15.5, E16.5, and newborn mice were removed and then the mice were fixed in 95% ethanol overnight at room temperature. Cartilage was stained with a 1.5% Alcian blue solution and bone tissues were subsequently stained with a 0.02% alizarin red solution. Skeletal samples were photographed under a stereoscopic microscope (Leica Microsystems)62.
In situ hybridization
Femurs of E12, E13.5, E15.5, and E16.5 mice were fixed in 4% PFA/PBS at 4 °C, washed with PBS, embedded in paraffin, and cut into 7 μm-thick sections. Digoxigenin (DIG)-labeled ssRNA probes were prepared using a DIG RNA Labeling Kit (Roche, Basel, Switzerland). Sections were deparaffinized and treated with 1 mg/ml proteinase K in 0.2 M HCl for 12 min at 37 °C and then subjected to acetylation in 0.1 M triethanolamine/0.25% acetic anhydride. After prehybridization, sections were incubated with DIG-labeled RNA probes at 65 °C for 16 h. The sections were further incubated with anti-DIG-AP Fab fragments (1:2500, Roche) for 2 h. After washing, sections were treated with nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (Roche) for various periods. We used a 405 bp fragment of mouse Col2a1 cDNA, a 637 bp fragment of mouse Col10a1 cDNA, a 579 bp fragment of mouse Ihh cDNA, a 779 bp fragment of mouse Pthr1 cDNA, a 634 bp fragment of mouse Runx2 cDNA, an 879 bp fragment of mouse Osterix cDNA, a 723 bp fragment of mouse Zfhx4 cDNA, a 516 bp fragment of mouse Osr1 cDNA, a 518 bp fragment of mouse Osr2 cDNA, and a 912 bp fragment of mouse Pax9 cDNA to generate anti-sense probes. The cDNA probes for Mmp1363, Sox921, Ihh21, Ppr9, and Osterix10 have been described previously. The cDNA probes for Col2a1 and Col10a1 were kindly provided by Noriyuki Tsumaki (Kyoto University, Kyoto, Japan) and used as described previously26. cDNA probes for Zfhx4 (Zfhx4-1 mRNA probe; Supplementary information) were synthesized using a DIG RNA Labeling Kit (Roche)64. For sections of palatal shelves and limb buds, the Zfhx4-2 mRNA probe (Supplementary information) was used.
von Kossa calcium staining
Sections were deparaffinized, incubated in a 5% silver nitrate solution for 30 min with exposure to sunlight, and then rinsed with two changes of distilled water. Sections were incubated in a 5% sodium thiosulfate solution for 2 min, rinsed with two changes of distilled water, and then incubated in a nuclear fast red solution for 5 min10.
Construction of expression vectors
Myc-tagged Osterix and Venus-tagged-Osterix expression vectors were used as described previously10. The Myc-Runx2 expression vector was generated by subcloning PCR-amplified, full-length Runx2 cDNA (variant 3, 1791 bp) into the EcoRI and XbaI sites of a pcDNA3 expression vector with six tandem repeats of the Myc tag at the N-terminal. Venus-tagged Runx2 was generated by subcloning the corresponding cDNA into the Venus-tagged expression vector. Flag-tagged Zfhx4 was generated using the Gateway Cloning System (Thermo Fisher Scientific)65. The PCR amplification products of Flag-tagged Zfhx4 cDNA were subcloned into the Kpnl and Xhol sites of the Gateway entry clone and the resulting clone was transferred into the Gateway pcDNA Zeo destination vector using LR Clonase enzyme mix, thereby constructing the Flag-tagged Zfhx4 expression vector66.
Transfection
Transfection of expression vectors was carried out using a X-treamGene 9 (Roche) in accordance with the manufacturer’s protocol.
Fluorochrome staining and immunocytochemical analysis
SW1353 cells were transfected with Flag-Zfhx4, Venus-Osterix, or Venus-Runx2 using the X-tremeGENE 9. After 48 h, cells were washed twice with PBS and fixed with 4% buffered paraformaldehyde (WAKO, Osaka, Japan) for 20 min. After treatment with 0.2% Triton X-100 in PBS for 5 min, cells were blocked with 1% BSA in PBS for 1 h, incubated with an anti-Flag (WAKO) antibody at room temperature for 2 h, and then incubated with Alexa Fluor 555-conjugated anti-mouse IgG (1:500, A21424; Thermo Fisher Scientific) for 30 min. Nuclear staining was performed using Vectashield with 4′,6-diamidino-2-phenylindole (Vector, Burlingame, CA, USA). Samples were visualized using a Leica TCS SP8 confocal microscope (Leica Microsystems).
Western blot analysis
Cells were washed with PBS and lysed in lysis buffer [20 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM ethylene glycol-bis[β-aminoethyl ether-N,N,N′,N′-tetraacetic acid, 1.5 mM MgCl2, 10% glycerol, 1% Triton X-100, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride, and 0.2 mM sodium orthovanadate]. Lysates were centrifuged at 12,000 rpm for 20 min at 4 °C and supernatants were boiled in sodium dodecyl sulfate (SDS) sample buffer with 0.5 M β-mercaptoethanol at 95 °C for 5 min. Protein samples were separated by SDS-polyacrylamide gel electrophoresis and then transferred to nitrocellulose membranes. After blocking with 5% BSA in 50 mM Tris-HCl buffer (pH 7.5), the nitrocellulose membranes were incubated with anti-Flag (1:10,000, WAKO), or anti-Myc (1:2000, #ab9132, Abcam) antibodies for 16 h at 4 °C, and then with anti-mouse (1:10,000, Medical and Biological Laboratories) or anti-goat (1:5000, Medical and Biological Laboratories) IgGs conjugated with horseradish peroxidase for 1 h at room temperature. Finally, membranes were visualized using an enhanced chemiluminescence detection reagent (ImmunoStar LD, WAKO)60.
Coimmunoprecipitation analysis
Cells transfected with Flag-Zfhx4, Myc-Osterix, or Myc-Runx2 were washed twice with ice-cold PBS and lysed in lysis buffer. Lysates were centrifuged at 12,000 rpm for 20 min at 4 °C and incubated with an anti-Flag antibody for 16 h at 4 °C, followed by immunoprecipitation with Dynabeads™ Protein G for Immunoprecipitation (Thermo Fisher Scientific). Immunoprecipitates were washed five times with ice-cold PBS and then boiled in SDS sample buffer with 0.5 M β-mercaptoethanol at 95 °C for 5 min. Supernatants were subsequently subjected to western blot analysis62.
Statistics and reproducibility
Randomization and blinding were not performed in the animal studies. Data were statistically analyzed using a two-tailed and unpaired Student’s t-test for intergroup comparisons. Values of P < 0.05 were considered statistically significant. All results were performed three times independently and reproduced with similar results.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Microarray data have been deposited in NCBI Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) under the accession code GSE126945. The source data for the graphs and charts in the main figures is available as Supplementary Data 1. The original uncropped images referring to Fig. 3a are provided in Supplementary Fig. 13. All other data are available from the corresponding author on reasonable request.
References
Kronenberg, H. M. Developmental regulation of the growth plate. Nature 423, 332–336 (2003).
Kobayashi, T. & Kronenberg, H. Minireview: transcriptional regulation in development of bone. Endocrinology 146, 1012–1017 (2005).
Long, F. Building strong bones: molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol. 13, 27–38 (2011).
Akiyama, H. & Lefebvre, V. Unraveling the transcriptional regulatory machinery in chondrogenesis. J. Bone Min. Metab. 29, 390–395 (2011).
Lefebvre, V., Huang, W., Harley, V. R., Goodfellow, P. N. & de Crombrugghe, B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol. Cell Biol. 17, 2336–2346 (1997).
Sekiya, I. et al. SOX9 enhances aggrecan gene promoter/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. J. Biol. Chem. 275, 10738–10744 (2000).
Kong, R. Y. et al. Intron-exon structure, alternative use of promoter and expression of the mouse collagen X gene, Col10a-1. Eur. J. Biochem. 213, 99–111 (1993).
Jimenez, M. J. et al. Collagenase 3 is a target of Cbfa1, a transcription factor of the runt gene family involved in bone formation. Mol. Cell Biol. 19, 4431–4442 (1999).
Inada, M. et al. Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev. Dyn. 214, 279–290 (1999).
Nishimura, R. et al. Osterix regulates calcification and degradation of chondrogenic matrices through matrix metalloproteinase 13 (MMP13) expression in association with transcription factor Runx2 during endochondral ossification. J. Biol. Chem. 287, 33179–33190 (2012).
de Crombrugghe, B., Lefebvre, V. & Nakashima, K. Regulatory mechanisms in the pathways of cartilage and bone formation. Curr. Opin. Cell Biol. 13, 721–727 (2001).
Bi, W., Deng, J. M., Zhang, Z., Behringer, R. R. & de Crombrugghe, B. Sox9 is required for cartilage formation. Nat. Genet. 22, 85–89 (1999).
Foster, J. W. et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372, 525–530 (1994).
Wagner, T. et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79, 1111–1120 (1994).
Benko, S. et al. Highly conserved non-coding elements on either side of SOX9 associated with Pierre Robin sequence. Nat. Genet. 41, 359–364 (2009).
Lefebvre, V., Li, P. & de Crombrugghe, B. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 17, 5718–5733 (1998).
Smits, P. et al. The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev. Cell 1, 277–290 (2001).
Smits, P., Dy, P., Mitra, S. & Lefebvre, V. Sox5 and Sox6 are needed to develop and maintain source, columnar, and hypertrophic chondrocytes in the cartilage growth plate. J. Cell Biol. 164, 747–758 (2004).
Akiyama, H., Chaboissier, M. C., Martin, J. F., Schedl, A. & de Crombrugghe, B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 16, 2813–2828 (2002).
Lefebvre, V. & Smits, P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res. C. Embryo Today 75, 200–212 (2005).
Yoshida, C. A. et al. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev. 18, 952–963 (2004).
Zheng, Q. et al. Type X collagen gene regulation by Runx2 contributes directly to its hypertrophic chondrocyte-specific expression in vivo. J. Cell Biol. 162, 833–842 (2003).
Zelzer, E. et al. Tissue specific regulation of VEGF expression during bone development requires Cbfa1/Runx2. Mech. Dev. 106, 97–106 (2001).
Kawakami, Y. et al. Transcriptional coactivator PGC-1alpha regulates chondrogenesis via association with Sox9. Proc. Nati Acad. Sci. USA 102, 2414–2419 (2005).
Furumatsu, T. et al. Sox9 and p300 cooperatively regulate chromatin-mediated transcription. J. Biol. Chem. 280, 35203–35208 (2005).
Hata, K. et al. Paraspeckle protein p54nrb links Sox9-mediated transcription with RNA processing during chondrogenesis in mice. J. Clin. Invest. 118, 3098–3108 (2008).
Amano, K. et al. Arid5a cooperates with Sox9 to stimulate chondrocyte-specific transcription. Mol. Biol. Cell 22, 1300–1311 (2011).
Hata, K. et al. Arid5b facilitates chondrogenesis by recruiting the histone demethylase Phf2 to Sox9-regulated genes. Nat. Commun. 4, 2850 (2013).
Takigawa, Y. et al. The transcription factor Znf219 regulates chondrocyte differentiation by assembling a transcription factory with Sox9. J. Cell Sci. 123, 3780–3788 (2010).
Zou, W. et al. The E3 ubiquitin ligase Wwp2 regulates craniofacial development through mono-ubiquitylation of Goosecoid. Nat. Cell Biol. 13, 59–65 (2011).
Nakamura, Y. et al. Wwp2 is essential for palatogenesis mediated by the interaction between Sox9 and mediator subunit 25. Nat. Commun. 2, 251 (2011).
Miller, J. et al. The core-binding factor beta subunit is required for bone formation and hematopoietic maturation. Nat. Genet. 32, 645–649 (2002).
Kundu, M. et al. Cbfbeta interacts with Runx2 and has a critical role in bone development. Nat. Genet. 32, 639–644 (2002).
Hata, K. et al. A CCAAT/enhancer binding protein beta isoform, liver-enriched inhibitory protein, regulates commitment of osteoblasts and adipocytes. Mol. Cell Biol. 25, 1971–1979 (2005).
Palomares, M. et al. Characterization of a 8q21.11 microdeletion syndrome associated with intellectual disability and a recognizable phenotype. Am. J. Hum. Genet. 89, 295–301 (2011).
Nishio, Y. et al. Runx2-mediated regulation of the zinc finger Osterix/Sp7 gene. Gene 372, 62–70 (2006).
Lan, Y. et al. Odd-skipped related 2 (Osr2) encodes a key intrinsic regulator of secondary palate growth and morphogenesis. Development 131, 3207–3216 (2004).
Gao, Y., Lan, Y., Ovitt, C. E. & Jiang, R. Functional equivalence of the zinc finger transcription factors Osr1 and Osr2 in mouse development. Dev. Biol. 328, 200–209 (2009).
Rice, R. et al. Disruption of Fgf10/Fgfr2b-coordinated epithelial-mesenchymal interactions causes cleft palate. J. Clin. Invest. 113, 1692–1700 (2004).
Satokata, I. & Maas, R. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat. Genet. 6, 348–356 (1994).
Zhang, Z. et al. Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development 129, 4135–4146 (2002).
Peters, H., Neubuser, A., Kratochwil, K. & Balling, R. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 12, 2735–2747 (1998).
Zhou, J., Gao, Y., Lan, Y., Jia, S. & Jiang, R. Pax9 regulates a molecular network involving Bmp4, Fgf10, Shh signaling and the Osr2 transcription factor to control palate morphogenesis. Development 140, 4709–4718 (2013).
Mori-Akiyama, Y., Akiyama, H., Rowitch, D. H. & de Crombrugghe, B. Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc. Nati Acad. Sci. USA 100, 9360–9365 (2003).
Aberg, T. et al. Phenotypic changes in dentition of Runx2 homozygote-null mutant mice. J. Histochem. Cytochem. 52, 131–139 (2004).
Komori, T. et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89, 755–764 (1997).
Davis, A. P., Witte, D. P., Hsieh-Li, H. M., Potter, S. S. & Capecchi, M. R. Absence of radius and ulna in mice lacking hoxa-11 and hoxd-11. Nature 375, 791–795 (1995).
Fromental-Ramain, C. et al. Specific and redundant functions of the paralogous Hoxa-9 and Hoxd-9 genes in forelimb and axial skeleton patterning. Development 122, 461–472 (1996).
Wellik, D. M. & Capecchi, M. R. Hox10 and Hox11 genes are required to globally pattern the mammalian skeleton. Science 301, 363–367 (2003).
Xu, B. & Wellik, D. M. Axial Hox9 activity establishes the posterior field in the developing forelimb. Proc. Nati Acad. Sci. USA 108, 4888–4891 (2011).
Neufeld, S. J., Wang, F. & Cobb, J. Genetic interactions between Shox2 and Hox genes during the regional growth and development of the mouse limb. Genetics 198, 1117–1126 (2014).
Bobick, B. E. & Cobb, J. Shox2 regulates progression through chondrogenesis in the mouse proximal limb. J. Cell Sci. 125, 6071–6083 (2012).
Panda, A., Gamanagatti, S., Jana, M. & Gupta, A. K. Skeletal dysplasias: a radiographic approach and review of common non-lethal skeletal dysplasias. World J. Radiol. 6, 808–825 (2014).
Horton, W. A., Hall, J. G. & Hecht, J. T. Achondroplasia. Lancet 370, 162–172 (2007).
Motley, A. M. et al. Mutational spectrum in the PEX7 gene and functional analysis of mutant alleles in 78 patients with rhizomelic chondrodysplasia punctata type 1. Am. J. Hum. Genet. 70, 612–624 (2002).
Chan, D., Taylor, T. K. & Cole, W. G. Characterization of an arginine 789 to cysteine substitution in alpha 1 (II) collagen chains of a patient with spondyloepiphyseal dysplasia. J. Biol. Chem. 268, 15238–15245 (1993).
Ido, A. et al. Cloning of the cDNA encoding the mouse ATBF1 transcription factor. Gene 168, 227–231 (1996).
Sun, X. et al. Heterozygous deletion of Atbf1 by the Cre-loxP system in mice causes preweaning mortality. Genesis 50, 819–827 (2012).
Yagi, T. et al. A novel ES cell line, TT2, with high germline-differentiating potency. Anal. Biochem. 214, 70–76 (1993).
Kida, J. et al. Interaction of LEF1 with TAZ is necessary for the osteoblastogenic activity of Wnt3a. Sci. Rep. 8, 10375 (2018).
Nogami, S. et al. ZFH4 protein is expressed in many neurons of developing rat brain. J. Comp. Neurol. 482, 33–49 (2005).
Yoshida, M. et al. The transcription factor Foxc1 is necessary for Ihh-Gli2-regulated endochondral ossification. Nat. Commun. 6, 6653 (2015).
Himeno, M. et al. Impaired vascular invasion of Cbfa1-deficient cartilage engrafted in the spleen. J. Bone Min. Res. 17, 1297–1305 (2002).
Gray, P. A. et al. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science 306, 2255–2257 (2004).
Freuler, F., Stettler, T., Meyerhofer, M., Leder, L. & Mayr, L. M. Development of a novel Gateway-based vector system for efficient, multiparallel protein expression in Escherichia coli. Protein Expr. Purif. 59, 232–241 (2008).
Chudnovsky, Y. et al. ZFHX4 interacts with the NuRD core member CHD4 and regulates the glioblastoma tumor-initiating cell state. Cell Rep. 6, 313–324 (2014).
Acknowledgements
We thank Dr. Atsushi Miyawaki (RIKEN, BSI) for the Venus construct. We also thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript. This study was supported in part by Japanese Ministry of Education, Culture, Sports, Science and Technology Grants-in-Aid for Scientific Research (R.N., 16H063930, 16H02686, 25293378, 20K204750, and 21H04841) and “Challenge to Intractable Oral Diseases” (E.N.).
Author information
Authors and Affiliations
Contributions
E.N. and R.N. directed the study. E.N., K.H., and M.A. designed and performed all in vitro and in vivo experiments. E.N., K.H., Y.T., M.A., S.K., and S.Y. performed the molecular and biochemical experiments. T.A. and M.K. generated mutant mice. H.A., N.S., and H.K. generated materials. E.N. assessed the data. E.N., M.A., H.K., T.I., T.Y., T.M., H.A., M.K., and S.N. discussed the data. E.N. and R.N. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Communications Biology thanks Hiroshi Asahara and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editors: Anam Akhtar. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakamura, E., Hata, K., Takahata, Y. et al. Zfhx4 regulates endochondral ossification as the transcriptional platform of Osterix in mice. Commun Biol 4, 1258 (2021). https://doi.org/10.1038/s42003-021-02793-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-021-02793-9
This article is cited by
-
A spatio-temporally constrained gene regulatory network directed by PBX1/2 acquires limb patterning specificity via HAND2
Nature Communications (2023)
-
Cigarette smoke extract impairs gingival epithelial barrier function
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.