Abstract
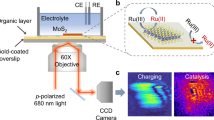
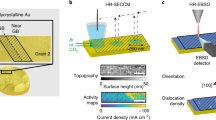
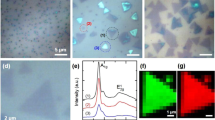
Understanding the structural evolution of individual active sites during a reaction is a long-standing target in surface science and catalysis. It is still challenging to precisely characterize in situ the intrinsic nature and evolution of the active site because the active site is too small for characterization techniques to decipher the local properties. Here we used electrochemical tip-enhanced Raman spectroscopy to monitor the geometric and electronic evolution of individual active sites of MoS2 during the hydrogen evolution reaction. Reconstruction regions of 40 nm with varied lattice and electron density from the edge to the nearby basal plane were observed during the hydrogen evolution reaction. We further revealed the progressive generation of active sites during the activation process. The synergistic reconstruction around edge due to the lattice deformation reduces the activation energy barriers and promotes the electrocatalytic reaction. These discoveries offer insights into our understanding of the active site and its dynamics during electrocatalysis.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data that support the findings of this study are available within the paper, the Supplementary Information and the Source data files. Additional data are available from the authors upon reasonable request. Source data are provided with this paper.
References
Zhao, S., Yang, Y. & Tang, Z. Insight into structural evolution, active sites, and stability of heterogeneous electrocatalysts. Angew. Chem. Int. Ed. 134, e202110186 (2022).
Li, H., Li, L. & Li, Y. The electronic structure and geometric structure of nanoclusters as catalytic active sites. Nanotechnol. Rev. 2, 515–528 (2013).
Mefford, J. T. et al. Correlative operando microscopy of oxygen evolution electrocatalysts. Nature 593, 67–73 (2021).
Nagashima, S. et al. Atomic-level observation of electrochemical platinum dissolution and redeposition. Nano Lett. 19, 7000–7005 (2019).
Roeffaers, M. B. J. et al. Spatially resolved observation of crystal-face-dependent catalysis by single turnover counting. Nature 439, 572–575 (2006).
Pfisterer, J. H. K., Liang, Y., Schneider, O. & Bandarenka, A. S. Direct instrumental identification of catalytically active surface sites. Nature 549, 74–77 (2017).
Sambur, J. B. et al. Sub-particle reaction and photocurrent mapping to optimize catalyst-modified photoanodes. Nature 530, 77–80 (2016).
Zeng, Z.-C. et al. Electrochemical tip-enhanced Raman spectroscopy. J. Am. Chem. Soc. 137, 11928–11931 (2015).
Kurouski, D., Mattei, M. & Van Duyne, R. P. Probing redox reactions at the nanoscale with electrochemical tip-enhanced Raman spectroscopy. Nano Lett. 15, 7956–7962 (2015).
Pfisterer, J. H. K., Baghernejad, M., Giuzio, G. & Domke, K. F. Reactivity mapping of nanoscale defect chemistry under electrochemical reaction conditions. Nat. Commun. 10, 5702 (2019).
Huang, S.-C. et al. Probing nanoscale spatial distribution of plasmonically excited hot carriers. Nat. Commun. 11, 4211 (2020).
Touzalin, T., Joiret, S., Lucas, I. T. & Maisonhaute, E. Electrochemical tip-enhanced Raman spectroscopy imaging with 8 nm lateral resolution. Electrochem. Commun. 108, 106557 (2019).
Cao, Y. Roadmap and direction toward high-performance MoS2 hydrogen evolution catalysts. ACS Nano 15, 11014–11039 (2021).
Deng, J. et al. Triggering the electrocatalytic hydrogen evolution activity of the inert two-dimensional MoS2 surface via single-atom metal doping. Energy Environ. Sci. 8, 1594–1601 (2015).
Jaramillo, T. F. et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 317, 100–102 (2007).
Huang, T.-X. et al. Probing the edge-related properties of atomically thin MoS2 at nanoscale. Nat. Commun. 10, 5544 (2019).
Novoselov, K. S. et al. Two-dimensional gas of massless Dirac fermions in graphene. Nature 438, 197–200 (2005).
Castellanos-Gomez, A. et al. Deterministic transfer of two-dimensional materials by all-dry viscoelastic stamping. 2D Mater. 1, 011002 (2013).
Bao, Y.-F. et al. Atomic force microscopy based top-illumination electrochemical tip-enhanced Raman spectroscopy. Anal. Chem. 92, 12548–12555 (2020).
Yang, L.-K. et al. Rational fabrication of a gold-coated AFM TERS tip by pulsed electrodeposition. Nanoscale 7, 18225–18231 (2015).
Mitterreiter, E. et al. In-situ visualization of hydrogen evolution sites on helium ion treated molybdenum dichalcogenides under reaction conditions. NPJ 2D Mater. Appl. 3, 25 (2019).
Mignuzzi, S. et al. Effect of disorder on Raman scattering of single-layer MoS2. Phys. Rev. B 91, 195411 (2015).
Sohier, T. et al. Enhanced electron–phonon interaction in multivalley materials. Phys. Rev. 9, 031019 (2019).
Wu, J.-B., Lin, M.-L., Cong, X., Liu, H.-N. & Tan, P.-H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 47, 1822–1873 (2018).
Zhang, X. et al. Phonon and Raman scattering of two-dimensional transition metal dichalcogenides from monolayer, multilayer to bulk material. Chem. Soc. Rev. 44, 2757–2785 (2015).
Chakraborty, B. et al. Symmetry-dependent phonon renormalization in monolayer MoS2 transistor. Phys. Rev. B 85, 161403 (2012).
Huang, Y., Nielsen, R. J., Goddard, W. A. & Soriaga, M. P. The reaction mechanism with free energy barriers for electrochemical dihydrogen evolution on MoS2. J. Am. Chem. Soc. 137, 6692–6698 (2015).
Li, W. et al. Hydrogen evolution reaction mechanism on 2H-MoS2 electrocatalyst. Appl. Surf. Sci. 498, 143869 (2019).
Zhu, C. R. et al. Strain tuning of optical emission energy and polarization in monolayer and bilayer MoS2. Phys. Rev. B 88, 121301 (2013).
Kim, J. et al. Anomalous optical excitations from arrays of whirlpooled lattice distortions in moiré superlattices. Nat. Mater. 21, 890–895 (2022).
Lunardon, M. et al. Catalytic activity of defect-engineered transition metal dichalcogenides mapped with atomic-scale precision by electrochemical scanning tunneling microscopy. ACS Energy Lett. 8, 972–980 (2023).
Singh, A., Sharma, G., Singh, B. P. & Vasa, P. Charge-induced lattice compression in monolayer MoS2. J. Phys. Chem. C 123, 17943–17950 (2019).
Lechner, C., Baranek, P. & Vach, H. Adsorption of atomic hydrogen on defect sites of graphite: Influence of surface reconstruction and irradiation damage. Carbon 127, 437–448 (2018).
Sun, J.-J. & Cheng, J. Solid-to-liquid phase transitions of sub-nanometer clusters enhance chemical transformation. Nat. Commun. 10, 5400 (2019).
DeRita, L. et al. Structural evolution of atomically dispersed Pt catalysts dictates reactivity. Nat. Mater. 18, 746–751 (2019).
Zugic, B. et al. Dynamic restructuring drives catalytic activity on nanoporous gold–silver alloy catalysts. Nat. Mater. 16, 558–564 (2017).
Hu, C. et al. In situ electrochemical production of ultrathin nickel nanosheets for hydrogen evolution electrocatalysis. Chem 3, 122–133 (2017).
Chia, X., Ambrosi, A., Sofer, Z., Luxa, J. & Pumera, M. Catalytic and charge transfer properties of transition metal dichalcogenides arising from electrochemical pretreatment. ACS Nano 9, 5164–5179 (2015).
Xi, F. et al. Structural transformation identification of sputtered amorphous MoSx as an efficient hydrogen-evolving catalyst during electrochemical activation. ACS Catal. 9, 2368–2380 (2019).
Li, G. et al. Activating MoS2 for pH-universal hydrogen evolution catalysis. J. Am. Chem. Soc. 139, 16194–16200 (2017).
Conley, H. J. et al. Bandgap engineering of strained monolayer and bilayer MoS2. Nano Lett. 13, 3626–3630 (2013).
Abidi, N., Bonduelle-Skrzypczak, A. & Steinmann, S. N. How stable are 2H-MoS2 edges under hydrogen dvolution reaction conditions? J. Phys. Chem. C 125, 17058–17067 (2021).
Hegner, M., Wagner, P. & Semenza, G. Ultralarge atomically flat template-stripped Au surfaces for scanning probe microscopy. Surf. Sci. 291, 39–46 (1993).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Togo, A. & Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 108, 1–5 (2015).
Steinmann, S. N. & Sautet, P. Assessing a first-principles model of an electrochemical interface by comparison with experiment. J. Phys. Chem. C 120, 5619–5623 (2016).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC) (grant numbers 22021001, 22227802, 21790354, 11874350, 92061118, 22372141 and 62374158), the National Key R&D Program of China (grant number 2023YFA1407000), the Strategic Priority Research Program of CAS (grant number XDB0460000), the Fundamental Research Funds for the Central Universities (grant number 20720220016) and the CAS Key Research Program of Frontier Sciences (grant numbers ZDBS-LY-SLH004 and XDPB22). We thank X. Yang for his assistance with theoretical discussion.
Author information
Authors and Affiliations
Contributions
T.-X.H., X.W. and B.R. conceived the project. T.-X.H., S.-S.W. and Y.-F.B. performed the experiments. M.-F.C. fabricated the SiO2-coated gold tip. X.C., J.-B.W., M.-L.L. and P.-H.T. performed the DFT calculations. L.W. helped with the experiments. T.-X.H., X.C., X.W. and B.R. wrote the manuscript. All authors participated in the analysis and discussion.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Patrick Unwin, Katrin F. Domke and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–20, Notes 1–5 and Tables 1 and 2.
Supplementary Data 1
Atomic coordinates of optimized computational models.
Source data
Source Data Fig. 1
Source data used to plot Fig. 1
Source Data Fig. 2
Source data used to plot Fig. 2
Source Data Fig. 3
Source data used to plot Fig. 3
Source Data Fig. 4
Source data used to plot Fig. 4
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, TX., Cong, X., Wu, SS. et al. Visualizing the structural evolution of individual active sites in MoS2 during electrocatalytic hydrogen evolution reaction. Nat Catal (2024). https://doi.org/10.1038/s41929-024-01148-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41929-024-01148-x