Abstract
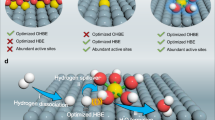
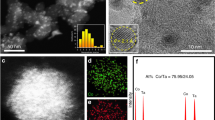
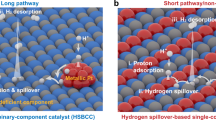
Constructing well-defined heterostructure interfaces in catalysts is an efficient strategy to break the so-called scaling relationships and to accelerate the reactions involving multiple intermediates. Here a cluster–cluster heterostructure catalyst composed of crystalline ruthenium cluster and amorphous chromium oxide cluster is designed to realize high-performance alkaline hydrogen electrocatalysis. The strongly coupled cluster–cluster heterostructure interface induces a unique interfacial interpenetration effect, which simultaneously optimizes the adsorption of intermediates on each cluster. The resulting catalyst exhibits impressive catalytic activities for the hydrogen oxidation reaction (exchange current density of 2.8 A mg−1Ru) and the hydrogen evolution reaction (mass activity of 23.0 A mg−1Ru at the overpotential of 100 mV) in alkaline media. The hydroxide exchange membrane fuel cell delivers a mass activity of 22.4 A mg−1Ru at 0.65 V and outstanding durability with no voltage loss over 105 h operation at 500 mA cm−2. The present work demonstrates the superiority of cluster–cluster heterostructure interface towards the development of advanced catalysts.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information files. All data is available from the authors upon reasonable request. The atomic coordinates of the computational models are given in Supplementary Data 1.
References
Glenk, G. & Reichelstein, S. Economics of converting renewable power to hydrogen. Nat. Energy 4, 216–222 (2019).
Staffell, I. et al. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 12, 463–491 (2019).
Stamenkovic, V. R., Strmcnik, D., Lopes, P. P. & Markovic, N. M. Energy and fuels from electrochemical interfaces. Nat. Mater. 16, 57–69 (2017).
Hu, Y. et al. Coplanar Pt/C nanomeshes with ultrastable oxygen reduction performance in fuel cells. Angew. Chem. Int. Ed. 60, 6533–6538 (2021).
Firouzjaie, H. A. & Mustain, W. E. Catalytic advantages, challenges, and priorities in alkaline membrane fuel cells. ACS Catal. 10, 225–234 (2020).
Varcoe, J. R. et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 7, 3135–3191 (2014).
Shi, L., Zhao, Y., Matz, S., Gottesfeld, S., Setzler, B. P. & Yan, Y. A shorted membrane electrochemical cell powered by hydrogen to remove CO2 from the air feed of hydroxide exchange membrane fuel cells. Nat. Energy 7, 238–247 (2022).
Li, C. & Baek, J.-B. The promise of hydrogen production from alkaline anion exchange membrane electrolyzers. Nano Energy 87, 106162 (2021).
Meyer, Q., Zeng, Y. & Zhao, C. In situ and operando characterization of proton exchange membrane fuel cells. Adv. Mater. 31, 1901900 (2019).
Kweon, D. H. et al. Ruthenium anchored on carbon nanotube electrocatalyst for hydrogen production with enhanced Faradaic efficiency. Nat. Commun. 11, 1278 (2020).
Cong, Y., Yi, B. & Song, Y. Hydrogen oxidation reaction in alkaline media: From mechanism to recent electrocatalysts. Nano Energy 44, 288–303 (2018).
Sheng, W., Gasteiger, H. A. & Shao-Horn, Y. Hydrogen oxidation and evolution reaction kinetics on platinum: acid vs alkaline electrolytes. J. Electrochem. Soc. 157, B1529 (2010).
Mahmood, J. et al. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction. Nat. Nano. 12, 441–446 (2017).
Xue, Y. et al. A highly-active, stable and low-cost platinum-free anode catalyst based on RuNi for hydroxide exchange membrane fuel cells. Nat. Commun. 11, 5651 (2020).
Zhang, J. et al. Engineering the near-surface of PtRu3 nanoparticles to improve hydrogen oxidation activity in alkaline electrolyte. Small 17, e2006698 (2021).
Wan, C., Zhang, Z. & Dong, J. et al. Amorphous nickel hydroxide shell tailors local chemical environment on platinum surface for alkaline hydrogen evolution reaction. Nat. Mater. 22, 1022–1029 (2023).
Sheng, W., Zhuang, Z., Gao, M., Zheng, J., Chen, J. G. & Yan, Y. Correlating hydrogen oxidation and evolution activity on platinum at different pH with measured hydrogen binding energy. Nat. Commun. 6, 5848 (2015).
Zhao, G., Rui, K., Dou, S. X. & Sun, W. Heterostructures for electrochemical hydrogen evolution reaction: a review. Adv. Funct. Mater. 28, 1803291 (2018).
Zhao, G., Li, P., Cheng, N., Dou, S. X. & Sun, W. An Ir/Ni(OH)2 heterostructured electrocatalyst for the oxygen evolution reaction: breaking the scaling relation, stabilizing iridium(V), and beyond. Adv. Mater. 32, 2000872 (2020).
Liang, Q. et al. Interfacing epitaxial dinickel phosphide to 2D nickel thiophosphate nanosheets for boosting electrocatalytic water splitting. ACS Nano 13, 7975–7984 (2019).
Feng, J.-X., Ye, S.-H., Xu, H., Tong, Y.-X. & Li, G.-R. Design and synthesis of FeOOH/CeO2 heterolayered nanotube electrocatalysts for the oxygen evolution reaction. Adv. Mater. 28, 4698–4703 (2016).
Zheng, X. et al. Multifunctional active-center-transferable platinum/lithium cobalt oxide heterostructured electrocatalysts towards superior water splitting. Angew. Chem. Int. Ed. 59, 14533–14540 (2020).
Lao, M. et al. Platinum/nickel bicarbonate heterostructures towards accelerated hydrogen evolution under alkaline conditions. Angew. Chem. Int. Ed. 58, 5432–5437 (2019).
Zhang, J., Zhang, Q. & Feng, X. Support and interface effects in water-splitting electrocatalysts. Adv. Mater. 31, 1808167 (2019).
Wu, G. et al. In-plane strain engineering in ultrathin noble metal nanosheets boosts the intrinsic electrocatalytic hydrogen evolution activity. Nat. Commun. 13, 4200 (2022).
Yang, Y. et al. Enhanced electrocatalytic hydrogen oxidation on Ni/NiO/C derived from a nickel-based metal–organic framework. Angew. Chem. Int. Ed. 58, 10644–10649 (2019).
Gerber, I. C. & Serp, P. A theory/experience description of support effects in carbon-supported catalysts. Chem. Rev. 120, 1250–1349 (2020).
Rong, H., Ji, S. & Zhang, J. et al. Synthetic strategies of supported atomic clusters for heterogeneous catalysis. Nat. Commun. 11, 5884 (2020).
Yang, F. et al. Boosting hydrogen oxidation activity of Ni in alkaline media through oxygen-vacancy-rich CeO2/Ni heterostructures. Angew. Chem. Int. Ed. 58, 14179–14183 (2019).
Zhou, Y. et al. Lattice-confined Ru clusters with high CO tolerance and activity for the hydrogen oxidation reaction. Nat. Catal. 3, 454–462 (2020).
Zheng, X. B., Li, B. B., Wang, Q. S., Wang, D. S. & Li, Y. D. Emerging low-nuclearity supported metal catalysts with atomic level precision for efficient heterogeneous catalysis. Nano Res. 15, 7806–7839 (2022).
Lin, J., Wang, W. & Li, G. Modulating surface/interface structure of emerging InGaN nanowires for efficient photoelectrochemical water splitting. Adv. Funct. Mater. 30, 2005677 (2020).
Zhang, B., Chen, Y., Wang, J., Pan, H. & Sun, W. Supported sub-nanometer clusters for electrocatalysis applications. Adv. Funct. Mater. 32, 2202227 (2022).
Zhang, B. et al. Atomically dispersed chromium coordinated with hydroxyl clusters enabling efficient hydrogen oxidation on ruthenium. Nat. Commun. 13, 5894 (2022).
Lu, B. Z. et al. Ruthenium atomically dispersed in carbon outperforms platinum toward hydrogen evolution in alkaline media. Nat. Commun. 10, 631 (2019).
Cui, W. W. et al. Cr(III) Adsorption by cluster formation on boehmite nanoplates in highly alkaline solution. Environ. Sci. Technol. 53, 11043–11055 (2019).
Jagminas, A., Niaura, G. & Žalnėravičius, R. et al. Laser light induced transformation of molybdenum disulphide-based nanoplatelet arrays. Sci. Rep. 6, 37514 (2016).
Gago, R., Vinnichenko, M., Hübner, R. & Redondo-Cubero, A. Bonding structure and morphology of chromium oxide films grown by pulsed-DC reactive magnetron sputter deposition. J. Alloy. Compd 672, 529–535 (2016).
Zhuang, Z. et al. Nickel supported on nitrogen-doped carbon nanotubes as hydrogen oxidation reaction catalyst in alkaline electrolyte. Nat. Commun. 7, 10141 (2016).
Singh, R. K. et al. Synthesis of CeOx‐decorated Pd/C catalysts by controlled surface reactions for hydrogen oxidation in anion exchange membrane fuel cells. Adv. Funct. Mater. 30, 2002087 (2020).
Cong, Y., Chai, C., Zhao, X., Yi, B. & Song, Y. Pt0.25Ru0.75/N–C as highly active and durable electrocatalysts toward alkaline hydrogen oxidation reaction. Adv. Mater. Inter. 7, 2000310 (2020).
Shen, L.-f et al. Does the oxophilic effect serve the same role for hydrogen evolution/oxidation reaction in alkaline media? Nano Energy 62, 601–609 (2019).
Ramaswamy, N., Ghoshal, S., Bates, M. K., Jia, Q., Li, J. & Mukerjee, S. Hydrogen oxidation reaction in alkaline media: relationship between electrocatalysis and electrochemical double-layer structure. Nano Energy 41, 765–771 (2017).
Lu, Y. et al. Tailoring competitive adsorption sites by oxygen-vacancy on cobalt oxides to enhance the electrooxidation of biomass. Adv. Mater. 34, 2107185 (2022).
Li, J. et al. A fundamental viewpoint on the hydrogen spillover phenomenon of electrocatalytic hydrogen evolution. Nat. Commun. 12, 3502 (2021).
Zhang, T., Yuan, B., Wang, W., He, J. & Xiang, X. Tailoring *H intermediate coverage on the CuAl2O4/CuO catalyst for enhanced electrocatalytic CO2 reduction to ethanol. Angew. Chem. Int. Ed. 62, e202302096 (2023).
Zhao, G., Jiang, Y., Dou, S.-X., Sun, W. & Pan, H. Interface engineering of heterostructured electrocatalysts towards efficient alkaline hydrogen electrocatalysis. Sci. Bull. 66, 85–96 (2021).
Zhu, S., Qin, X. & Xiao, F. et al. The role of ruthenium in improving the kinetics of hydrogen oxidation and evolution reactions of platinum. Nat. Catal. 4, 711–718 (2021).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 27, 1787–1799 (2006).
Andersen, H. C. Molecular dynamics simulations at constant pressure and/or temperature. J. Chem. Phys. 72, 2384 (1980).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Atkins, P., De Paula, J. & Keeler, J. Atkins’ Physical Chemistry (Oxford University Press, 2017).
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (no. 2022YFB4002503), the National Natural Science Foundation of China (no. 92261119 and 52171224), the Natural Science Foundation of Zhejiang Province (grant no. LZ22B030006), the Research Grant Council of the Hong Kong Special Administrative Region (C6011-20G), the Innovation and Technology Commission of the Hong Kong Special Administrative Region (no. ITC-CNERC14EG03). B.Z. acknowledges support from China Postdoctoral Science Foundation (no. 2021M692757), the National Postdoctoral Program for Innovative Talents (no. BX2021251) and Zhejiang Provincial Natural Science Foundation (no. LQ23E010005). The authors thank beamline BL11B and BL14W1 of the Shanghai Synchrotron Radiation Facility (SSRF) for providing the XAFS beamtime, and Photoemission End-Station (BL10B) at the National Synchrotron Radiation Laboratory (NSRL) for NEXAFS beamtime.
Author information
Authors and Affiliations
Contributions
B.Z. performed the catalyst development, characterizations and performance evaluation. J.W., D.L., Y.C. and J.C. helped in electrocatalysis experiments and material characterizations. G.L. and C.M.W. helped in fuel cell test. L.X. and P. Z. helped in DFT calculations. HP., M.G., Y.L., Y.Y., M.S., W.S. and B.Z. analysed the data. All the authors contributed to the overall scientific discussions and edited the manuscript. W.S., H.P. and B.Z. conceived the idea and co-wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Ligang Feng, Marion Giraud and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–44, Tables 1–11, notes and References 1–60.
Supplementary Data 1
Atomic coordinates of the initial and final configurations of the trajectories in AIMD simulations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, B., Wang, J., Liu, G. et al. A strongly coupled Ru–CrOx cluster–cluster heterostructure for efficient alkaline hydrogen electrocatalysis. Nat Catal 7, 441–451 (2024). https://doi.org/10.1038/s41929-024-01126-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-024-01126-3