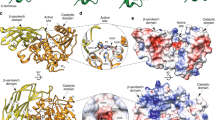
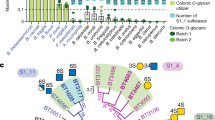
Abstract
Microbes utilize polysaccharides to protect their surfaces and build biofilms, whereas metazoans employ large mucins densely decorated with O-glycans to protect surfaces and keep microbes at a distance. However, gut microbes in mucus also feed on host mucins, thus imposing a need for continuous renewal to maintain protection, clearance and mucus homeostasis. Glycopeptidases that can cleave mucins are known, but mucinases that specifically cleave mucins are not. Here we report the discovery of such microbial mucinases that cleave mucins with trimmed glycans, recognize dense clusters of O-glycans, and employ a structural fold and catalytic machinery reminiscent of glycan hydrolases and peptidases. These di-glutamate mucinases are also found in eukaryotes, and we propose that they are designed to clear mucins following scavenging of O-glycans to promote healthy gut–microbiome homeostasis.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The crystal structure of HC7ΔCBM-apo, as well as those of the HC7ΔCBM-E200A–triglycopeptide and HC7ΔCBM-E200A–tetraglycopeptide complexes, have been deposited at the RCSB PDB with accession codes 8PMU, 8PN5 and 8PN3, respectively. Previously published PDB structures used in this study are available under accession codes 4QHX, 1ZM1, 7ZVB, 2IFR, 1U0A, 1Y43 and 3ZSJ. Data files of the classical MD simulation and QM/MM metadynamics simulations have been deposited in Zenodo (https://doi.org/10.5281/zenodo.10103923). The full uncollapsed tree is available as Supplementary Data on Zenodo (https://doi.org/10.5281/zenodo.10103923) as well as the raw treefile used to generate this figure. Other data are available from the corresponding author upon request. The kinetics and computational data generated in this study are provided as source data. Any other data may be provided by the corresponding authors on request. Source data are provided with this paper.
References
Hansson, G. C. Mucins and the microbiome. Annu. Rev. Biochem. 89, 769–793 (2020).
Hansson, G. C. Mucus and mucins in diseases of the intestinal and respiratory tracts. J. Intern. Med. 285, 479–490 (2019).
Belzer, C. Nutritional strategies for mucosal health: the interplay between microbes and mucin glycans. Trends Microbiol. 30, 13–21 (2021).
Birchenough, G., Schroeder, B. O., Backhed, F. & Hansson, G. C. Dietary destabilisation of the balance between the microbiota and the colonic mucus barrier. Gut Microbes 10, 246–250 (2019).
Johansson, M. E., Sjovall, H. & Hansson, G. C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 10, 352–361 (2013).
Bell, A. & Juge, N. Mucosal glycan degradation of the host by the gut microbiota. Glycobiology 31, 691–696 (2021).
Liang, Q. et al. Sialic acid plays a pivotal role in licensing Citrobacter rodentium’s transition from the intestinal lumen to a mucosal adherent niche. Proc. Natl Acad. Sci. USA 120, e2301115120 (2023).
Wu, C. M. et al. Mucin glycans drive oral microbial community composition and function. NPJ Biofilms Microbiomes 9, 11 (2023).
Wheeler, K. M. et al. Mucin glycans attenuate the virulence of Pseudomonas aeruginosa in infection. Nat. Microbiol. 4, 2146–2154 (2019).
Werlang, C. A. et al. Mucin O-glycans suppress quorum-sensing pathways and genetic transformation in Streptococcus mutans. Nat. Microbiol. 6, 574–583 (2021).
Wang, B. X. et al. Host-derived O-glycans inhibit toxigenic conversion by a virulence-encoding phage in Vibrio cholerae. EMBO J. 42, e111562 (2023).
Kesimer, M. & Sheehan, J. K. Mass spectrometric analysis of mucin core proteins. Methods Mol. Biol. 842, 67–79 (2012).
Shon, D. J., Kuo, A., Ferracane, M. J. & Malaker, S. A. Classification, structural biology and applications of mucin domain-targeting proteases. Biochem. J. 478, 1585–1603 (2021).
Shon, D. J. et al. An enzymatic toolkit for selective proteolysis, detection and visualization of mucin-domain glycoproteins. Proc. Natl Acad. Sci. USA 117, 21299–21307 (2020).
Taleb, V. et al. Structural and mechanistic insights into the cleavage of clustered O-glycan patches-containing glycoproteins by mucinases of the human gut. Nat. Commun. 13, 4324 (2022).
Nason, R. et al. Display of the human mucinome with defined O-glycans by gene engineered cells. Nat. Commun. 12, 4070 (2021).
Bustos, N. A., Ribbeck, K. & Wagner, C. E. The role of mucosal barriers in disease progression and transmission. Adv. Drug Deliv. Rev. 200, 115008 (2023).
Ince, D., Lucas, T. M. & Malaker, S. A. Current strategies for characterization of mucin-domain glycoproteins. Curr. Opin. Chem. Biol. 69, 102174 (2022).
Konstantinidi, A. et al. Exploring the glycosylation of mucins by use of O-glycodomain reporters recombinantly expressed in glycoengineered HEK293 cells. J. Biol. Chem. 298, 101784 (2022).
Pluvinage, B. et al. Architecturally complex O-glycopeptidases are customized for mucin recognition and hydrolysis. Proc. Natl Acad. Sci. USA 118, e2019220118 (2021).
Birch, P. R. Targeted differential display of abundantly expressed sequences from the basidiomycete Phanerochaete chrysosporium which contain regions coding for fungal cellulose-binding domains. Curr. Genet. 33, 70–76 (1998).
Hemsworth, G. R., Henrissat, B., Davies, G. J. & Walton, P. H. Discovery and characterization of a new family of lytic polysaccharide monooxygenases. Nat. Chem. Biol. 10, 122–126 (2014).
Drula, E. et al. The carbohydrate-active enzyme database: functions and literature. Nucleic Acids Res. 50, D571–D577 (2022).
Rawlings, N. D. et al. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 46, D624–D632 (2018).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Sheikh, A. et al. Enterotoxigenic Escherichia coli degrades the host MUC2 mucin barrier to facilitate critical pathogen-enterocyte interactions in human small intestine. Infect. Immun. 90, e0057221 (2022).
Patel, S. A critical review on serine protease: key immune manipulator and pathology mediator. Allergol. Immunopathol. (Madr.) 45, 579–591 (2017).
Kollman, P. Free energy calculations: applications to chemical and biochemical phenomena. Chem. Rev. 93, 2395–2417 (1993).
Sanz-Martinez, I., Pereira, S., Merino, P., Corzana, F. & Hurtado-Guerrero, R. Molecular recognition of GalNAc in mucin-type O-glycosylation. Acc. Chem. Res. 56, 548–560 (2023).
Corzana, F. et al. Serine versus threonine glycosylation: the methyl group causes a drastic alteration on the carbohydrate orientation and on the surrounding water shell. J. Am. Chem. Soc. 129, 9458–9467 (2007).
Corzana, F. et al. New insights into α-GalNAc-Ser motif: influence of hydrogen bonding versus solvent interactions on the preferred conformation. J. Am. Chem. Soc. 128, 14640–14648 (2006).
Invernizzi, M. & Parrinello, M. Exploration vs convergence speed in adaptive-bias enhanced sampling. J. Chem. Theory Comput. 18, 3988–3996 (2022).
Sondergaard, C. R., Olsson, M. H., Rostkowski, M. & Jensen, J. H. Improved treatment of ligands and coupling effects in empirical calculation and rationalization of pKa values. J. Chem. Theory Comput. 7, 2284–2295 (2011).
Holm, L. Dali server: structural unification of protein families. Nucleic Acids Res. 50, W210–W215 (2022).
Gaiser, O. J. et al. Structural basis for the substrate specificity of a Bacillus 1,3-1,4-β-glucanase. J. Mol. Biol. 357, 1211–1225 (2006).
Tsai, L. C., Shyur, L. F., Cheng, Y. S. & Lee, S. H. Crystal structure of truncated Fibrobacter succinogenes 1,3-1,4-β-d-glucanase in complex with β-1,3-1,4-cellotriose. J. Mol. Biol. 354, 642–651 (2005).
Pillai, B. et al. Crystal structure of scytalidoglutamic peptidase with its first potent inhibitor provides insights into substrate specificity and catalysis. J. Mol. Biol. 365, 343–361 (2007).
Del Amo-Maestro, L. et al. Molecular and in vivo studies of a glutamate-class prolyl-endopeptidase for coeliac disease therapy. Nat. Commun. 13, 4446 (2022).
Yabuki, Y., Kubota, K., Kojima, M., Inoue, H. & Takahashi, K. Identification of a glutamine residue essential for catalytic activity of aspergilloglutamic peptidase by site-directed mutagenesis. FEBS Lett. 569, 161–164 (2004).
Sasaki, H. et al. The crystal structure of an intermediate dimer of aspergilloglutamic peptidase that mimics the enzyme-activation product complex produced upon autoproteolysis. J. Biochem. 152, 45–52 (2012).
Chandra, N. R., Prabu, M. M., Suguna, K. & Vijayan, M. Structural similarity and functional diversity in proteins containing the legume lectin fold. Protein Eng. 14, 857–866 (2001).
Potter, S. C. et al. HMMER web server: 2018 update. Nucleic Acids Res. 46, W200–W204 (2018).
Undheim, E. A. B. & Jenner, R. A. Phylogenetic analyses suggest centipede venom arsenals were repeatedly stocked by horizontal gene transfer. Nat. Commun. 12, 818 (2021).
Haitjema, C. H. et al. A parts list for fungal cellulosomes revealed by comparative genomics. Nat. Microbiol. 2, 17087 (2017).
Wang, Y. et al. Molecular dating of the emergence of anaerobic rumen fungi and the impact of laterally acquired genes. mSystems 4, e00247-19 (2019).
Pedram, K. et al. Lysosomal cathepsin D mediates endogenous mucin glycodomain catabolism in mammals. Proc. Natl Acad. Sci. USA 119, e2117105119 (2022).
Varki, A. Selectin ligands. Proc. Natl Acad. Sci. USA 91, 7390–7397 (1994).
Cohen, M. & Varki, A. Modulation of glycan recognition by clustered saccharide patches. Int. Rev. Cell Mol. Biol. 308, 75–125 (2014).
Lathem, W. W. et al. StcE, a metalloprotease secreted by Escherichia coli O157:H7, specifically cleaves C1 esterase inhibitor. Mol. Microbiol. 45, 277–288 (2002).
Rillahan, C. D. & Paulson, J. C. Glycan microarrays for decoding the glycome. Annu. Rev. Biochem. 80, 797–823 (2011).
Murin, C. D. et al. Structure of 2G12 Fab2 in complex with soluble and fully glycosylated HIV-1 Env by negative-stain single-particle electron microscopy. J. Virol. 88, 10177–10188 (2014).
Daniels, C. N. & Saunders, K. O. Antibody responses to the HIV-1 envelope high mannose patch. Adv. Immunol. 143, 11–73 (2019).
Lee, C. D. et al. A cross-neutralizing antibody between HIV-1 and influenza virus. PLoS Pathog. 17, e1009407 (2021).
Chen, Y. H. et al. The GAGOme: a cell-based library of displayed glycosaminoglycans. Nat. Methods 15, 881–888 (2018).
Narimatsu, Y. et al. Genetic glycoengineering in mammalian cells. J. Biol. Chem. 296, 100448 (2021).
Grys, T. E., Siegel, M. B., Lathem, W. W. & Welch, R. A. The StcE protease contributes to intimate adherence of enterohemorrhagic Escherichia coli O157:H7 to host cells. Infect. Immun. 73, 1295–1303 (2005).
Luis, A. S. & Hansson, G. C. Intestinal mucus and their glycans: a habitat for thriving microbiota. Cell Host Microbe 31, 1087–1100 (2023).
Kumar, P. et al. EatA, an immunogenic protective antigen of enterotoxigenic Escherichia coli, degrades intestinal mucin. Infect. Immun. 82, 500–508 (2014).
Pedram, K. et al. Design of a mucin-selective protease for targeted degradation of cancer-associated mucins. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-01840-6 (2023).
Schjoldager, K. T., Narimatsu, Y., Joshi, H. J. & Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 21, 729–749 (2020).
Pham, D. et al. Epidemiology, modern diagnostics and the management of mucorales infections. J. Fungi (Basel) 9, 659 (2023).
Hoenigl, M. et al. The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe 3, e543–e552 (2022).
Smith, C. & Lee, S. C. Current treatments against mucormycosis and future directions. PLoS Pathog. 18, e1010858 (2022).
Jones, P. G., Hammell, K. L., Gettinby, G. & Revie, C. W. Detection of emamectin benzoate tolerance emergence in different life stages of sea lice, Lepeophtheirus salmonis, on farmed Atlantic salmon, Salmo salar L. J. Fish Dis. 36, 209–220 (2013).
Khalturin, K. et al. Transgenic stem cells in Hydra reveal an early evolutionary origin for key elements controlling self-renewal and differentiation. Dev. Biol. 309, 32–44 (2007).
Siebert, S. et al. Stem cell differentiation trajectories in Hydra resolved at single-cell resolution. Science 365, eaav9314 (2019).
Scappaticci, A. A. Jr, Kahn, F. & Kass-Simon, G. Nematocyst discharge in Hydra vulgaris: differential responses of desmonemes and stenoteles to mechanical and chemical stimulation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 157, 184–191 (2010).
Bull, C., Joshi, H. J., Clausen, H. & Narimatsu, Y. Cell-based glycan arrays—a practical guide to dissect the human glycome. STAR Protoc. 1, 100017 (2020).
Narimatsu, Y. et al. An atlas of human glycosylation pathways enables display of the human glycome by gene engineered cells. Mol. Cell 75, 394–407 (2019).
Plattner, C., Hofener, M. & Sewald, N. One-pot azidochlorination of glycals. Org. Lett. 13, 545–547 (2011).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Tunyasuvunakool, K. et al. Highly accurate protein structure prediction for the human proteome. Nature 596, 590–596 (2021).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Maier, J. A. et al. ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 11, 3696–3713 (2015).
Kirschner, K. N. et al. GLYCAM06: a generalizable biomolecular force field. Carbohydrates. J. Comput. Chem. 29, 622–655 (2008).
Salomon-Ferrer, R., Gotz, A. W., Poole, D., Le Grand, S. & Walker, R. C. Routine microsecond molecular dynamics simulations with AMBER on GPUs. 2. Explicit solvent particle mesh Ewald. J. Chem. Theory Comput. 9, 3878–3888 (2013).
Ryckaert, J.-P., Ciccotti, G. & Berendsen, H. J. C. Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comput. Phys. 23, 327–341 (1977).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: an N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Warshel, A. & Levitt, M. Theoretical studies of enzymic reactions: dielectric, electrostatic and steric stabilization of the carbonium ion in the reaction of lysozyme. J. Mol. Biol. 103, 227–249 (1976).
Kuhne, T. D. et al. CP2K: an electronic structure and molecular dynamics software package—Quickstep: efficient and accurate electronic structure calculations. J. Chem. Phys. 152, 194103 (2020).
Tribello, G. A., Bonomi, M., Branduardi, D., Camilloni, C. & Bussi, G. PLUMED 2: new feathers for an old bird. Comput. Phys. Commun. 185, 604–613 (2014).
VandeVondele, J. et al. Quickstep: fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 167, 103–128 (2005).
Goedecker, S., Teter, M. & Hutter, J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B Condens. Matter 54, 1703–1710 (1996).
Marcos-Alcalde, I., Lopez-Vinas, E. & Gomez-Puertas, P. MEPSAnd: minimum energy path surface analysis over n-dimensional surfaces. Bioinformatics 36, 956–958 (2020).
Acknowledgements
This work was supported by the Novo Nordisk Foundation (NNF22OC0076899 to H.J.J., NNF20SA0067193 to B.H. and NNF210071658 to H.C.), the Danish National Research Foundation (DNRF107), the Lundbeck Foundation, the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 787684 (to C.B.), the Spanish Ministry of Science, Innovation and Universities (BFU2016-75633-P, PID2019-105451GB-100, PID2022-136362NB-100, PID2021-127622OB-100, PID2020-118893GB-100 and CEX2021-001202-M), Gobierno de Aragón (E34_R17 and LMP58_18) with FEDER (2014–2020) funds for ‘Building Europe from Aragón’ for financial support (to R.H.-G.), and the Agency for Management of University and Research Grants of Catalonia (AGAUR, 2021-SGR-00680 to C.R.). We thank ALBA (Barcelona, Spain) synchrotron beamline XALOC, and Red Española de Supercomputación (RES-BSC) for computer time in CTE-Power and MareNostrum IV. Q.L. thanks the EU for a Marie-Skłodowska Curie fellowship (MCSA-IF-2020, agreement no. 101025071). R.H.-G. thanks M. del Carmen Hurtado-Lago for help with the design of Fig. 5c.
Author information
Authors and Affiliations
Contributions
Y.N., C.B., B.H., H.C., H.J.J. and R.H.-G. conceived and directed the study. Y.N. and C.B. performed most of the experimental analysis. B.H., H.J.J. and L.H. performed the bioinformatics. F.D. and R.V. contributed to recombinant expression and purification. V.T. and D.S.-N. expressed and purified the HC7ΔCBM and Mucor mucinases. V.T. crystallized the HC7ΔCBM and HC7ΔCBM-E200A, and R.H.-G. solved and built the crystal structures. F.C. performed the MD simulations. Q.L. and C.R. performed MD and QM/MM enhanced sampling simulations. I.C. synthetized the glycopeptides. Y.N., H.C. and R.H.-G. wrote the paper, and all authors edited and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The University of Copenhagen has filed a patent application on the cell-based display platform (US20190330601A1). GlycoDisplay Aps, Copenhagen, Denmark, has obtained a licence to the field of the patent application. Y.N. and H.C. are co-founders of GlycoDisplay Aps and hold ownership in the company. The other authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Elisa Fadda and Shinya Fushinobu for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–28, Tables 1 and 2 and Methods.
Supplementary Data
Supplementary source data.
Source data
Source Data Figs. 1c, 2b,c,e,f, 3a and 4e
Data used to generate the graph.
Source Data Figs. 2d and 4d
Unprocessed gel.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Narimatsu, Y., Büll, C., Taleb, V. et al. A family of di-glutamate mucin-degrading enzymes that bridges glycan hydrolases and peptidases. Nat Catal 7, 386–400 (2024). https://doi.org/10.1038/s41929-024-01116-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-024-01116-5
This article is cited by
-
Taking a walk to find new mucinases
Nature Catalysis (2024)