Abstract
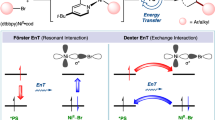
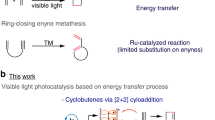
The photochemical [2+2] cycloaddition of styrenes provides a frequently used route for the synthesis of multi-substituted cyclobutanes. Despite the extensive studies in noble-metal and organo-photocatalysis, developing sustainable cycloaddition methods with copper photosensitizers is still in its infancy, largely owing to their low reactivity and photostability. Here we show that the introduction of a binap-ligated heteroleptic copper(I) complex to the linker of a microporous zirconium-based metal−organic framework produces a highly stable and reusable heterogeneous photocatalyst with an extended excited-state lifetime. Under visible light irradiation, this robust copper triplet photosensitizer efficiently promotes multiple intermolecular crossed [2+2] cycloadditions, including an underdeveloped cycloaddition reaction of simple styrenes with electron-deficient alkenes. Our findings suggest that metal–organic framework-based heterogenization strategies have the potential to advance copper photocatalysis and foster a variety of visible light-mediated energy-transfer processes.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are included in this manuscript and Source data or are available from the corresponding author upon reasonable request. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2240330 (Cu-1) and 2244329 (Cu-3). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
References
Goetzke, F. W., Hell, A. M. L., van Dijk, L. & Fletcher, S. P. A catalytic asymmetric cross-coupling approach to the synthesis of cyclobutanes. Nat. Chem. 13, 880–886 (2021).
Dembitsky, V. M. Naturally occurring bioactive cyclobutane-containing (CBC) alkaloids in fungi, fungal endophytes, and plants. Phytomedicine 21, 1559–1581 (2014).
Namyslo, J. C. & Kaufmann, D. E. The application of cyclobutane derivatives in organic synthesis. Chem. Rev. 103, 1485–1538 (2003).
Wang, M. & Lu, P. Catalytic approaches to assemble cyclobutane motifs in natural product synthesis. Org. Chem. Front. 5, 254–259 (2018).
Poplata, S., Tröster, A., Zou, Y.-Q. & Bach, T. Recent advances in the synthesis of cyclobutanes by olefin [2+2] photocycloaddition reactions. Chem. Rev. 116, 9748–9815 (2016).
Zhou, Q.-Q., Zou, Y.-Q., Lu, L.-Q. & Xiao, W.-J. Visible-light-induced organic photochemical reactions through energy-transfer pathways. Angew. Chem. Int. Ed. 58, 1586–1604 (2019).
Sicignano, M., Rodríguez, R. I. & Alemán, J. Recent visible light and metal free strategies in [2+2] and [4+2] photocycloadditions. Eur. J. Org. Chem. 2021, 3303–3321 (2021).
Zhu, M., Zhang, X., Zheng, C. & You, S.-L. Energy-transfer-enabled dearomative cycloaddition reactions of indoles/pyrroles via excited-state aromatics. Acc. Chem. Res. 55, 2510–2525 (2022).
Ischay, M. A., Ament, M. S. & Yoon, T. P. Crossed intermolecular [2+2] cycloaddition of styrenes by visible light photocatalysis. Chem. Sci. 3, 2807–2811 (2012).
Riener, M. & Nicewicz, D. A. Synthesis of cyclobutane lignans via an organic single electron oxidant-electron relay system. Chem. Sci. 4, 2625–2629 (2013).
Das, S. et al. Asymmetric counteranion-directed photoredox catalysis. Science 379, 494–499 (2023).
Tanaka, K. et al. Redox potential controlled selective oxidation of styrenes for regio- and stereoselective crossed intermolecular [2 + 2] cycloaddition via organophotoredox catalysis. Org. Lett. 22, 5207–5211 (2020).
Li, R. et al. Photocatalytic regioselective and stereoselective [2 + 2] cycloaddition of styrene derivatives using a heterogeneous organic photocatalyst. ACS Catal. 7, 3097–3101 (2017).
Piane, J. J. et al. Organic photoredox-catalyzed cycloadditions under single-chain polymer confinement. ACS Catal. 10, 13251–13256 (2020).
Lu, Z. & Yoon, T. P. Visible light photocatalysis of [2+2] styrene cycloadditions by energy transfer. Angew. Chem. Int. Ed. 51, 10329–10332 (2012).
Liu, Z. et al. Aggregation-enabled intermolecular photo[2+2]cycloaddition of aryl terminal olefins by visible-light catalysis. CCS Chem. 2, 582–588 (2020).
Murray, P. R. D. et al. Intermolecular crossed [2 + 2] cycloaddition promoted by visible-light triplet photosensitization: expedient access to polysubstituted 2-oxaspiro[3.3]heptanes. J. Am. Chem. Soc. 143, 4055–4063 (2021).
Jiang, Y., Wang, C., Rogers, C. R., Kodaimati, M. S. & Weiss, E. A. Regio- and diastereoselective intermolecular [2+2] cycloadditions photocatalysed by quantum dots. Nat. Chem. 11, 1034–1040 (2019).
Jiang, Y., López-Arteaga, R. & Weiss, E. A. Quantum dots photocatalyze intermolecular [2 + 2] cycloadditions of aromatic alkenes adsorbed to their surfaces via van der Waals interactions. J. Am. Chem. Soc. 144, 3782–3786 (2022).
Hernandez-Perez, A. C. & Collins, S. K. Heteroleptic Cu-based sensitizers in photoredox catalysis. Acc. Chem. Res. 49, 1557–1565 (2016).
Forero Cortés, P. A., Marx, M., Trose, M. & Beller, M. Heteroleptic copper complexes with nitrogen and phosphorus ligands in photocatalysis: overview and perspectives. Chem. Catal. 1, 298–338 (2021).
Brégent, T., Bouillon, J.-P. & Poisson, T. Copper-photocatalyzed contra-thermodynamic isomerization of polarized alkenes. Org. Lett. 22, 7688–7693 (2020).
Cruché, C., Neiderer, W. & Collins, S. K. Heteroleptic copper-based complexes for energy-transfer processes: E → Z isomerization and tandem photocatalytic sequences. ACS Catal. 11, 8829–8836 (2021).
Rogge, S. M. et al. Metal–organic and covalent organic frameworks as single-site catalysts. Chem. Soc. Rev. 46, 3134–3184 (2017).
Wei, Y.-S., Zhang, M., Zou, R. & Xu, Q. Metal–organic framework-based catalysts with single metal sites. Chem. Rev. 120, 12089–12174 (2020).
Chen, W. et al. Site-isolated azobenzene-containing metal–organic framework for cyclopalladated catalyzed Suzuki–Miyuara coupling in flow. ACS Appl. Mater. Interfaces 13, 51849–51854 (2021).
Ma, B. et al. Metal–organic framework supported copper photoredox catalysts for iminyl radical-mediated reactions. Angew. Chem. Int. Ed. 62, e202300233 (2023).
Liu, J. et al. MOF-enabled confinement and related effects for chemical catalyst presentation and utilization. Chem. Soc. Rev. 51, 1045–1097 (2022).
Zhou, H.-C., Long, J. R. & Yaghi, O. M. Introduction to metal–organic frameworks. Chem. Rev. 112, 673–674 (2012).
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal-organic frameworks. Science 341, 1230444 (2013).
Cavka, J. H. et al. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 130, 13850–13851 (2008).
Newsome, W. J. et al. Solid state multicolor emission in substitutional solid solutions of metal–organic frameworks. J. Am. Chem. Soc. 141, 11298–11303 (2019).
Kalaj, M. & Cohen, S. M. Postsynthetic modification: an enabling technology for the advancement of metal–organic frameworks. ACS Cent. Sci. 6, 1046–1057 (2020).
Li, J. et al. Self-adaptive dual-metal-site pairs in metal-organic frameworks for selective CO2 photoreduction to CH4. Nat. Catal. 4, 719–729 (2021).
Ma, X. et al. Modulating coordination environment of single-atom catalysts and their proximity to photosensitive units for boosting MOF photocatalysis. J. Am. Chem. Soc. 143, 12220–12229 (2021).
Wang, C., Xie, Z., deKrafft, K. E. & Lin, W. Doping metal–organic frameworks for water oxidation, carbon dioxide reduction, and organic photocatalysis. J. Am. Chem. Soc. 133, 13445–13454 (2011).
Wang, C., deKrafft, K. E. & Lin, W. Pt nanoparticles@photoactive metal–organic frameworks: efficient hydrogen evolution via synergistic photoexcitation and electron injection. J. Am. Chem. Soc. 134, 7211–7214 (2012).
Zhang, X. et al. Catalytic chemoselective functionalization of methane in a metal–organic framework. Nat. Catal. 1, 356–362 (2018).
Pi, Y. et al. Metal−organic frameworks integrate Cu photosensitizers and secondary building unit-supported Fe catalysts for photocatalytic hydrogen evolution. J. Am. Chem. Soc. 142, 10302–10307 (2020).
Kong, X.-J., Lin, Z., Zhang, Z.-M., Zhang, T. & Lin, W. Hierarchical integration of photosensitizing metal–organic frameworks and nickel-containing polyoxometalates for efficient visible-light-driven hydrogen evolution. Angew. Chem. Int. Ed. 55, 6411–6416 (2016).
Coote, S. C. & Bach, T. Enantioselective intermolecular [2+2] photocycloadditions of isoquinolone mediated by a chiral hydrogen-bonding template. J. Am. Chem. Soc. 135, 14948–14951 (2013).
Mejía, E. et al. A noble-metal-free system for photocatalytic hydrogen production from water. Chem. Eur. J. 19, 15972–15978 (2013).
Ischay, M. A., Lu, Z. & Yoon, T. P. [2+2] cycloadditions by oxidative visible light photocatalysis. J. Am. Chem. Soc. 132, 8572–8574 (2010).
Lei, T. et al. General and efficient intermolecular [2+2] photodimerization of chalcones and cinnamic acid derivatives in solution through visible-light catalysis. Angew. Chem. Int. Ed. 56, 15407–15410 (2017).
Zhu, M., Zheng, C., Zhang, X. & You, S.-L. Synthesis of cyclobutane-fused angular tetracyclic spiroindolines via visible-light-promoted intramolecular dearomatization of indole derivatives. J. Am. Chem. Soc. 141, 2636–2644 (2019).
Girvin, Z. C. et al. Asymmetric photochemical [2 + 2]-cycloaddition of acyclic vinylpyridines through ternary complex formation and an uncontrolled sensitization mechanism. J. Am. Chem. Soc. 144, 20109–20117 (2022).
Yu, X. & Cohen, S. M. Photocatalytic metal–organic frameworks for selective 2,2,2-trifluoroethylation of styrenes. J. Am. Chem. Soc. 138, 12320–12323 (2016).
Fan, Y., Zheng, H., Labalme, S. & Lin, W. Molecular engineering of metal–organic layers for sustainable tandem and synergistic photocatalysis. J. Am. Chem. Soc. 145, 4158–4165 (2023).
Ni, T., Caldwell, R. A. & Melton, L. A. The relaxed and spectroscopic energies of olefin triplets. J. Am. Chem. Soc. 111, 457–464 (1989).
He, J., Shao, Q., Wu, Q. & Yu, J.-Q. Pd(II)-catalyzed enantioselective C(sp3)–H borylation. J. Am. Chem. Soc. 139, 3344–3347 (2017).
Keller, S. et al. Luminescent copper(I) complexes with bisphosphane and halogen-substituted 2,2′-bipyridine ligands. Dalton Trans. 47, 14263–14276 (2018).
Roth, H. G., Romero, N. A. & Nicewicz, D. A. Experimental and calculated electrochemical potentials of common organic molecules for applications to single-electron redox chemistry. Synlett 27, 714–723 (2016).
Cheng, X., Li, T., Liu, Y. & Lu, Z. Stereo- and enantioselective benzylic C–H alkenylation via photoredox/nickel dual catalysis. ACS Catal. 11, 11059–11065 (2021).
Shen, Y. et al. Room-temperature photosynthesis of propane from CO2 with Cu single atoms on vacancy-rich TiO2. Nat. Commun. 14, 1117 (2023).
Acknowledgements
The authors thank The University of Hong Kong, the Research Grants Council of the Hong Kong Special Administrative Region, People’s Republic of China (grant nos. 27301820 and 17313922 to J.H., 27200822 and 16302520 to P.C.Y.C., and 15301521 and 15300819 to T.W.B.L.), the Croucher Foundation, the Innovation and Technology Commission (HKSAR, China), and the National Natural Science Foundation of China (grant no. 22201236 to J.H. and 22172136 to T.W.B.L.) for their financial support. The authors thank C.-M. Che for help and discussions.
Author information
Authors and Affiliations
Contributions
J.H. supervised the project and designed the experiments. J.G. developed the photocatalytic cycloadditions and expanded the substrate scope with the help of W.Y.T. Q.X., Z.L. and X.W. prepared and characterized the catalytic materials. L.-J.L. and K.-H.L. analysed the crystal structures of the heteroleptic copper complexes. W.-P.T., H.-X.S. and P.C.Y.C. measured the solid-state emission spectra. T.W.B.L. simulated the EXAFS spectra. J.H. wrote the manuscript with the contributions from J.G., Q.X. and L.-J.L.
Corresponding author
Ethics declarations
Competing interests
J.H., J.G. and Q.X. are listed as co-inventors of a US patent application, ‘Heterogeneous catalysts and methods making and using thereof’ (63/604,642). The patent describes the synthesis of heterogeneous copper photosensitizers and the photocatalytic performance presented in this manuscript. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Jose Aleman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, Figs. 1–45, Tables 1–10 and references.
Supplementary Data 1
Crystallographic data for complex Cu-1.
Supplementary Data 2
Crystallographic data for complex Cu-3.
Source data
Source Data Fig. 2
Raw data for Fig. 2c–e.
Source Data Fig. 6
Raw data for Fig. 6b,c.
Source Data Fig. 7
Raw data for Fig. 7a.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, J., Xia, Q., Tang, W.Y. et al. Visible light-mediated intermolecular crossed [2+2] cycloadditions using a MOF-supported copper triplet photosensitizer. Nat Catal 7, 307–320 (2024). https://doi.org/10.1038/s41929-024-01112-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-024-01112-9