Abstract
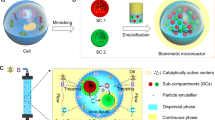
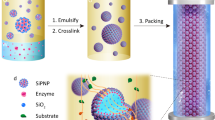
Integration of multiple incompatible catalysts in one continuous-flow reaction system is of paramount significance for green synthetic chemistry. Here we develop a continuous-flow cascade catalytic system by co-packing micrometre-sized Pickering emulsion droplets and solid microspheres in a column reactor. This co-packed system can not only provide the optimum microenvironments for each incompatible catalyst but also enables them to work synergistically through directional transfer of reaction intermediates. The success of the concept is based on a phenomenon found here, namely coexistence of Pickering emulsion droplets and solid microspheres without droplet disruption even under flow and high pressure due to specific interfacial effects. As proof of concept, two chemoenzymatic cascade reactions for synthesis of chiral cyanohydrins and chiral esters were examined; both these reactions exhibited 7-fold to 77-fold enhanced catalysis efficiency compared with their batch reactions, 99% enantioselectivity and long-term operational stability (240 h).

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Schrittwieser, J. H., Velikogne, S., Hall, M. & Kroutil, W. Artificial biocatalytic linear cascades for preparation of organic molecules. Chem. Rev. 118, 270–348 (2018).
Vázquez-González, M., Wang, C. & Willner, I. Biocatalytic cascades operating on macromolecular scaffolds and in confined environments. Nat. Catal. 3, 256–273 (2020).
Sperl, J. M. & Sieber, V. Multienzyme cascade reactions—status and recent advances. ACS Catal. 8, 2385–2396 (2018).
Wang, Y. X., Zhao, Q. C., Haag, R. & Wu, C. Z. Biocatalytic synthesis using self-assembled polymeric nano- and microreactors. Angew. Chem. Int. Ed. 61, e202213974 (2022).
Isaacs, M. A. et al. A spatially orthogonal hierarchically porous acid–base catalyst for cascade and antagonistic reactions. Nat. Catal. 3, 921–931 (2020).
Rudroff, F. et al. Opportunities and challenges for combining chemo- and biocatalysis. Nat. Catal. 1, 12–22 (2018).
Alonso, S. et al. Genetically engineered proteins with two active sites for enhanced biocatalysis and synergistic chemo- and biocatalysis. Nat. Catal. 3, 319–328 (2020).
Li, X. Y. et al. Highly active enzyme–metal nanohybrids synthesized in protein–polymer conjugates. Nat. Catal. 2, 718–725 (2019).
Wang, N. M. et al. Chemical recycling of polyethylene by tandem catalytic conversion to propylene. J. Am. Chem. Soc. 144, 18526–18531 (2022).
Britton, J. & Raston, C. L. Multi-step continuous-flow synthesis. Chem. Soc. Rev. 46, 1250–1271 (2017).
Jiao, J. et al. Multi-step continuous-flow organic synthesis: opportunities and challenges. Chem. Eur. J. 27, 4817–4838 (2021).
Contente, M. L. & Paradisi, F. Self-sustaining closed-loop multienzyme mediated conversion of amines into alcohols in continuous reactions. Nat. Catal. 1, 452–459 (2018).
Tsubogo, T., Oyamada, H. & Kobayashi, S. Multi-step continuous-flow synthesis of (R)- and (S)-rolipram using heterogeneous catalysts. Nature 520, 329–332 (2015).
Yasukawa, T., Masuda, R. & Kobayashi, S. Development of heterogeneous catalyst systems for the continuous synthesis of chiral amines via asymmetric hydrogenation. Nat. Catal. 2, 1088–1092 (2019).
Sagmeister, P. et al. Advanced real-time process analytics for multi-step synthesis in continuous flow. Angew. Chem. Int. Ed. 60, 8139–8148 (2021).
Wang, H. et al. Biomimetic enzyme cascade reaction system in microfluidic electrospray microcapsules. Sci. Adv. 4, eaat2816 (2018).
Russell, M. G. & Jamison, T. F. Seven-step continuous-flow synthesis of linezolid without intermediate purification. Angew. Chem. Int. Ed. 58, 7678–7681 (2019).
Sahoo, H. R., Kralj, J. G. & Jensen, K. F. Multi-step continuous-flow microchemical synthesis involving multiple reactions and separations. Angew. Chem. Int. Ed. 46, 5704–5708 (2007).
Cole-Hamilton, D. J. Homogeneous catalysis—new approaches to catalyst separation, recovery, and recycling. Science 299, 1702–1706 (2003).
Yu, T. et al. Recent advances in continuous-flow enantioselective catalysis. Chem. Eur. J. 26, 5729–5747 (2020).
Altava, B., Burguete, M. I., García-Verdugo, E. & Luis, S. V. Chiral catalysts immobilized on achiral polymers: effect of the polymer support on the performance of the catalyst. Chem. Soc. Rev. 47, 2722–2771 (2018).
Borah, P., Fianchini, M. & Pericàs, M. A. Assessing the role of site isolation and compartmentalization in packed-bed flow reactors for processes involving wolf-and-lamb scenarios. ACS Catal. 11, 6234–6242 (2021).
Wang, M. et al. Combining Pd nanoparticles on MOFs with cross-linked enzyme aggregates of lipase as powerful chemoenzymatic platform for one-pot dynamic kinetic resolution of amines. J. Catal. 378, 153–163 (2019).
Saito, Y., Nishizawa, K., Laroche, B., Ishitani, H. & Kobayashi, S. Continuous-flow synthesis of (R)-tamsulosin utilizing sequential heterogeneous catalysis. Angew. Chem. Int. Ed. 61, e202115643 (2022).
Li, Z. L. et al. Highly selective conversion of carbon dioxide to aromatics over tandem catalysts. Joule 3, 570–583 (2019).
Ichitsuka, T., Takahashi, I., Koumura, N., Sato, K. & Kobayashi, S. Continuous synthesis of aryl amines from phenols utilizing integrated packed-bed flow systems. Angew. Chem. Int. Ed. 59, 15891–15896 (2020).
Durndell, L. J. et al. Cascade aerobic selective oxidation over contiguous dual-catalyst beds in continuous flow. ACS Catal. 9, 5345–5352 (2019).
Mattey, A. P. et al. Development of continuous-flow systems to access secondary amines through previously incompatible biocatalytic cascades. Angew. Chem. Int. Ed. 60, 18660–18665 (2021).
Zhang, M. et al. Compartmentalized droplets for continuous-flow liquid–liquid interface catalysis. J. Am. Chem. Soc. 138, 10173–10183 (2016).
Zhang, M. et al. Ionic liquid-droplet microreactor for catalysis reactions not at equilibrium. J. Am. Chem. Soc. 139, 17387–17396 (2017).
Zhang, M. et al. Pickering emulsion droplet-based biomimetic microreactors for continuous-flow cascade reactions. Nat. Commun. 13, 475 (2022).
Zhao, H., Ran, R., Wang, L., Li, C. S. & Zhang, S. J. Novel continuous process for methacrolein production in numerous droplet reactors. AIChE J. 66, e16239 (2020).
Zhang, H., Shang, M. J., Zhao, Y. C. & Su, Y. H. Process intensification of 2,2′-(4-nitrophenyl) dipyrromethane synthesis with a SO3H-functionalized ionic liquid catalyst in Pickering-emulsion-based packed-bed microreactors. Micromachines 12, 796 (2021).
Wu, H., Du, X., Meng, X., Qiu, D. & Qiao, Y. A three-tiered colloidosomal microreactor for continuous-flow catalysis. Nat. Commun. 12, 6113 (2021).
Tang, X. P. et al. Heteropoly acids-functionalized Janus particles as catalytic emulsifier for heterogeneous acylation in flow ionic liquid-in-oil Pickering emulsion. Colloid Surf. A 570, 191–198 (2019).
Crossley, S., Faria, J., Shen, M. & Resasco, D. E. Solid nanoparticles that catalyze biofuel upgrade reactions at the water/oil interface. Science 327, 68–72 (2010).
Ni, L., Yu, C., Wei, Q. B., Liu, D. M. & Qiu, J. S. Pickering emulsion catalysis: interfacial chemistry, catalyst design, challenges, and perspectives. Angew. Chem. Int. Ed. 61, e202115885 (2022).
Chang, F. Q., Vis, C. M., Ciptonugroho, W. & Bruijnincx, P. C. A. Recent developments in catalysis with Pickering emulsions. Green Chem. 23, 2575–2594 (2021).
Chen, Z. W. et al. Design of surface-active artificial enzyme particles to stabilize Pickering emulsions for high-performance biphasic biocatalysis. Adv. Mater. 28, 1682–1688 (2016).
Dedovets, D. et al. Multiphase microreactors based on liquid–liquid and gas–liquid dispersions stabilized by colloidal catalytic particles. Angew. Chem. Int. Ed. 61, e202107537 (2022).
Sun, Z., Glebe, U., Charan, H., Bçker, A. & Wu, C. Z. Enzyme–polymer conjugates as robust Pickering interfacial biocatalysts for efficient biotransformations and one‐pot cascade reactions. Angew. Chem. Int. Ed. 57, 13810–13814 (2018).
Schoonen, L. & van Hest, J. C. M. Compartmentalization approaches in soft matter science: from nanoreactor development to organelle mimics. Adv. Mater. 28, 1109–1128 (2016).
Jiang, S., da Silva, L. C., Ivanov, T., Mottola, M. & Landfester, K. Synthetic silica nano‐organelles for regulation of cascade reactions in multi‐compartmentalized systems. Angew. Chem. Int. Ed. 61, e202113784 (2022).
Deng, N. N., Yelleswarapu, M. & Huck, W. T. S. Monodisperse uni- and multicompartment liposomes. J. Am. Chem. Soc. 138, 7584–7591 (2016).
Jiang, H. et al. Engineering hybrid microgels as particulate emulsifiers for reversible Pickering emulsions. Chem. Sci. 13, 39–43 (2022).
Zhang, Q. H., Zhang, S. G. & Deng, Y. Q. Recent advances in ionic liquid catalysis. Green Chem. 13, 2619–2637 (2011).
Aveyard, R., Binks, B. P. & Clint, J. H. Emulsions stabilised solely by colloidal particles. Adv. Colloid Interface Sci. 100−102, 503–546 (2003).
Wei, L. J., Yan, S., Wang, H. H. & Yang, H. Q. Fabrication of multi-compartmentalized mesoporous silica microspheres through a Pickering droplet strategy for enhanced CO2 capture and catalysis. NPG Asia Mater. 10, 899–911 (2018).
Wu, T. et al. Multi-body coalescence in Pickering emulsions. Nat. Commun. 6, 5929 (2015).
Eral, H. B., Manukyan, G. & Oh, J. M. Wetting of a drop on a sphere. Langmuir 27, 5340–5346 (2011).
Wingstrand, E., Laurell, A., Fransson, L., Hult, K. & Moberg, C. Minor enantiomer recycling: metal catalyst, organocatalyst and biocatalyst working in concert. Chem. Eur. J. 15, 12107–12113 (2009).
Lundgren, S., Wingstrand, E., Penhoat, M. & Moberg, C. Dual Lewis acid–Lewis base activation in enantioselective cyanation of aldehydes using acetyl cyanide and cyanoformate as cyanide sources. J. Am. Chem. Soc. 127, 11592–11593 (2005).
Brunel, J. M. & Holmes, I. P. Chemically catalyzed asymmetric cyanohydrin syntheses. Angew. Chem. Int. Ed. 43, 2752–2778 (2004).
Denard, C. A., Hartwig, J. F. & Zhao, H. M. Multi-step one-pot reactions combining biocatalysts and chemical catalysts for asymmetric synthesis. ACS Catal. 3, 2856–2864 (2013).
Kim, J. H. & Kim, G. J. Enantioselectivities of chiral Ti(IV) salen complexes immobilized on MCM-41 in asymmetric trimethylsilylcyanation of benzaldehyde. Catal. Lett. 92, 123–130 (2004).
Zhu, Y. Z., Fow, K. L., Chuah, G. K. & Jaenicke, S. Dynamic kinetic resolution of secondary alcohols combining enzyme-catalyzed transesterification and zeolite-catalyzed racemization. Chem. Eur. J. 13, 541–547 (2007).
Lozano, P., De Diego, T., Larnicol, M., Vaultier, M. & Iborra, J. L. Chemoenzymatic dynamic kinetic resolution of rac-1-phenylethanol in ionic liquids and ionic liquids/supercritical carbon dioxide systems. Biotechnol. Lett. 28, 1559–1565 (2006).
Lozano, P. et al. Long-term continuous chemoenzymatic dynamic kinetic resolution of rac-1-phenylethanol using ionic liquids and supercritical carbon dioxide. Green Chem. 11, 538–542 (2009).
Acknowledgements
This work is supported by the Natural Science Foundation of China (21925203, 22332002 and 21902093), the National Key Research and Development Program of China (2021YFC2101900), the Natural Science Research Foundation of Shanxi Province (202303021211016), the 100 Talent Project of Shanxi Province, the Fund for Shanxi ‘1331 Project’ and the Foundation of State Key Laboratory of Coal Conversion (grant number J24-25-909).
Author information
Authors and Affiliations
Contributions
H.Y. conceived and supervised the project. M.Z. executed the experiments and collected the data. T.L., J.Y and L.D. helped M.Z. to conduct part of the experiments. R.E. conducted the theoretical calculations. N.X. contributed to preparing some of the figures. B.P.B. and F.C. suggested varying the hydrophobicity of the SMs and analyzed the findings. H.Y. and M.Z. wrote the paper. All authors edited the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Yuanhai Su, Ana Jurinjak Tusek and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–26, Methods, mass spectrometry data and references.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, M., Ettelaie, R., Li, T. et al. Pickering emulsion droplets and solid microspheres acting synergistically for continuous-flow cascade reactions. Nat Catal 7, 295–306 (2024). https://doi.org/10.1038/s41929-024-01110-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-024-01110-x