Abstract
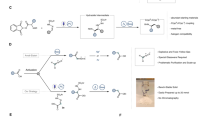
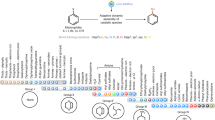
Transition metal-catalysed cross-coupling is a versatile tool for the construction of (hetero)biaryl scaffolds. However, the cross-electrophile coupling using abundant (hetero)aryl halides and pseudohalides is still in its infancy. In particular, a robust and general method for the cross-electrophile coupling would allow unparalleled entry into the vast collection of commercially available, structurally diverse (hetero)aryl halides and pseudohalides as coupling partners. Here we demonstrate a ligand-controlled visible light-driven mono-metallic cross-electrophile coupling platform in which the synergistic operation of a dual palladium catalytic cycle differentiates the electrophiles based on the bond dissociation enthalpy. This method is mild and selective and displays efficacy towards a wide range of functional groups and challenging heteroaryls. The power of the transformation has been revealed through the synthesis of (hetero)biaryl cores of various pharmaceuticals and diversification of peptides. Bypassing the traditional transmetalation step, this technology enables a general strategy for the cross-electrophile coupling of (hetero)aryl halides and pseudohalides.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data relating to materials and methods, experimental procedures, mechanistic studies, characterization data and NMR spectra are available in Supplementary Information. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2220229 (L80), 2220165 (complex E) and 2286772 (complex F). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Bringmann, G., Günther, C., Ochse, M., Schupp, O. & Tasler, S. Biaryls in Nature: A Multi-Faceted Class of Stereochemically, Biosynthetically, and Pharmacologically Intriguing Secondary Metabolites (Springer, 2001).
Hajduk, P. J., Bures, M., Praestgaard, J. & Fesik, S. W. Privileged molecules for protein binding identified from NMR-based screening. J. Med. Chem. 43, 3443 (2000).
Wu, J. S., Cheng, S. W., Cheng, Y. J. & Hsu, C. S. Donor–acceptor conjugated polymers based on multifused ladder-type arenes for organic solar cells. Chem. Soc. Rev. 44, 1113–1154 (2015).
Kaes, C., Katz, A. & Hosseini, M. W. Bipyridine: the most widely used ligand. a review of molecules comprising at least two 2,2′-bipyridine units. Chem. Rev. 100, 3553–3590 (2000).
Hansen, E. C. et al. New ligands for nickel catalysis from diverse pharmaceutical heterocycle libraries. Nat. Chem. 8, 1126–1130 (2016).
Meijere, A. D. & Diederich, F. Metal-Catalyzed Cross-Coupling Reactions 2nd edn, Vol. 1 (Wiley-VCH, 2004).
Magano, J. & Dunetz, J. R. Large-scale applications of transition metal-catalyzed couplings for the synthesis of pharmaceuticals. Chem. Rev. 111, 2177–2250 (2011).
Brown, D. G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
García-Melchor, M., Braga, A. A., Lledós, A., Ujaque, G. & Maseras, F. Computational perspective on Pd-catalyzed C–C crosscoupling reaction mechanisms. Acc. Chem. Res. 46, 2626–2634 (2013).
Cox, P. A., Leach, A. G., Campbell, A. D. & Lloyd-Jones, G. C. Protodeboronation of heteroaromatic, vinyl, and cyclopropyl boronic acids: pH–rate profiles, autocatalysis, and disproportionation. J. Am. Chem. Soc. 138, 9145–9157 (2016).
Cox, P. A. et al. Base-catalyzed aryl-B(OH)2 protodeboronation revisited: from concerted proton transfer to liberation of a transient aryl anion. J. Am. Chem. Soc. 139, 13156–13165 (2017).
Cook, X. A. F., de Gombert, A., McKnight, J., Pantaine, L. R. E. & Willis, M. C. The 2-pyridyl problem: challenging nucleophiles in cross-coupling arylations. Angew. Chem. Int. Ed. 60, 11068–11091 (2021).
Knapp, D. M., Gillis, E. P. & Burke, M. D. A general solution for unstable boronic acids: slow-release cross-coupling from air-stable MIDA boronates. J. Am. Chem. Soc. 131, 6961–6963 (2009).
Markovic, T., Rocke, B. N., Blakemore, D. C., Mascitti, V. & Willis, M. C. Pyridine sulfinates as general nucleophilic coupling partners in palladium-catalyzed cross-coupling reactions with aryl halides. Chem. Sci. 8, 4437–4442 (2017).
Alberico, D., Scott, M. E. & Lautens, M. Aryl–aryl bond formation by transition metal-catalyzed direct arylation. Chem. Rev. 107, 174–238 (2007).
Grover, J., Prakash, G., Goswami, N. & Maiti, D. Traditional and sustainable approaches for the construction of C–C bonds by harnessing C–H arylation. Nat. Commun. 13, 1085 (2022).
Yang, Y. D., Lan, J. B. & You, J. S. Oxidative C–H/C–H coupling reactions between two (hetero)arenes. Chem. Rev. 117, 8787–8863 (2017).
Zhou, M., Tsien, J. & Qin, T. Sulfur(IV)-mediated unsymmetrical heterocycle cross-couplings. Angew. Chem. Int. Ed. 59, 7372–7376 (2020).
Hilton, M. C. et al. Heterobiaryl synthesis by contractive C–C coupling via P(V) intermediates. Science 362, 799–804 (2018).
Everson, D. A. & Weix, D. J. Cross-electrophile coupling: principles of reactivity and selectivity. J. Org. Chem. 79, 4793–4798 (2014).
Hassan, J., Sévignon, M., Gozzi, C., Schulz, E. & Lemaire, M. Aryl–aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. 102, 1359–1470 (2002).
Khan, F., Dlugosch, M., Liu, X. & Banwell, M. G. The palladium-catalyzed Ullmann cross-coupling reaction: a modern variant on a time-honored process. Acc. Chem. Res. 51, 1784–1795 (2018).
Chow, W. K., So, C. M., Lau, C. P. & Kwong, F. Y. Palladium-catalyzed borylation of aryl mesylates and tosylates and their applications in one-pot sequential Suzuki–Miyaura biaryl synthesis. Chem. Eur. J. 17, 6913–6917 (2011).
Molander, G. A., Trice, S. L. J., Kennedy, S. M., Dreher, S. D. & Tudge, M. T. Scope of the palladium-catalyzed aryl borylation utilizing bis-boronic acid. J. Am. Chem. Soc. 134, 11667–11673 (2012).
Knappke, C. E. I. et al. Reductive cross-coupling reactions between two electrophiles. Chem. Eur. J. 20, 6828–6842 (2014).
Tang, J. et al. Chemoselective cross-coupling between two different and unactivated C(aryl)–O bonds enabled by chromium catalysis. J. Am. Chem. Soc. 142, 7715–7720 (2020).
Yi, L., Ji, T., Chen, K.-Q., Chen, X.-Y. & Rueping, M. Nickel-catalyzed reductive cross-couplings: new opportunities for carbon–carbon bond formations through photochemistry and electrochemistry. CCS Chem. 4, 9–30 (2022).
Ackerman, L. K. G., Lovell, M. M. & Weix, D. J. Multimetallic catalysed cross-coupling of aryl bromides with aryl triflates. Nature 524, 454–457 (2015).
Huang, L., Ackerman, L. K. G., Kang, K., Parsons, A. M. & Weix, D. J. LiCl-accelerated multimetallic cross-coupling of aryl chlorides with aryl triflates. J. Am. Chem. Soc. 141, 10978–10983 (2019).
Kang, K., Huang, L. & Weix, D. J. Sulfonate versus sulfonate: nickel and palladium multimetallic cross-electrophile coupling of aryl triflates with aryl tosylates. J. Am. Chem. Soc. 142, 10634–10640 (2020).
Kang, K., Loud, N. L., DiBenedetto, T. A. & Weix, D. J. A general, multimetallic cross-Ullmann biheteroaryl synthesis from heteroaryl halides and heteroaryl triflates. J. Am. Chem. Soc. 143, 21484–21491 (2021).
Wu, T.-F., Zhang, Y.-J., Fu, Y., Liu, F.-J., Tang, J.-T., Liu, P., Toste, F. D. & Ye, B. Zirconium-redox-shuttled cross-electrophile coupling of aromatic and heteroaromatic halides. Chem 7, 1963–1974 (2021).
Chuentragool, P., Kurandina, D. & Gevorgyan, V. Catalysis with palladium complexes photoexcited by visible light. Angew. Chem. Int. Ed. 58, 11586–11598 (2019).
Zhou, W.-J., Cao, G.-M., Zhang, Z.-P. & Yu, D.-G. Visible light-induced palladium-catalysis in organic synthesis. Chem. Lett. 48, 181–191 (2019).
Torres, G. M., Liu, Y. & Arndtsen, B. A. A dual light-driven palladium catalyst: breaking the barriers in carbonylation reactions. Science 368, 318–323 (2020).
Ratushnyy, M., Kvasovs, N., Sarkar, S. & Gevorgyan, V. Visible light‐induced palladium‐catalyzed generation of aryl radicals from aryl triflates. Angew. Chem. Int. Ed. 59, 10316–10320 (2020).
Marchese, A. D. et al. Pd(0)/blue light-promoted carboiodination-reaction evidence for reversible C–I bond formation via a radical pathway. J. Am. Chem. Soc. 144, 20554–20560 (2022).
Bellotti, P., Koy, M., Gutheil, C., Heuvela, S. & Glorius, F. Three-component three-bond forming cascade via palladium photoredox catalysis. Chem. Sci. 12, 1810–1817 (2021).
Kancherla, R. et al. Oxidative addition to palladium(0) made easy through photoexcited-state metal catalysis: experiment and computation. Angew. Chem. Int. Ed. 58, 3412–3416 (2019).
Luo, Y.-R. Comprehensive Handbook of Chemical Bond Energies (CRC Press, 2007).
Chilamari, M., Immel, J. R., Chen, P.-H., Alghafli, B. M. & Bloom, S. Flavin metallaphotoredox catalysis: synergistic synthesis in water. ACS Catal. 12, 4175–4181 (2022).
Zhou, W.-J. et al. Visible-light-driven palladium-catalyzed radical alkylation of C–H bonds with unactivated alkyl bromides. Angew. Chem. Int. Ed. 56, 15683–15687 (2017).
Martin, R. & Buchwald, S. L. Palladium-catalyzed Suzuki–Miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands. Acc. Chem. Res. 41, 1461–1473 (2008).
Kim, S.-H. & Rieke, R. D. 2-Pyridyl and 3-pyridylzinc bromides: direct preparation and coupling reaction. Tetrahedron 66, 3135–3146 (2010).
O’Neill, B. T., Yohannes, D., Bundesmann, M. W. & Arnold, E. P. Total synthesis of (±)-cytisine. Org. Lett. 2, 4201–4204 (2000).
Reichert, E. C., Feng, K., Sather, A. C. & Buchwald, S. L. Pd-catalyzed amination of base-sensitive five-membered heteroaryl halides with aliphatic amines. J. Am. Chem. Soc. 145, 3323–3329 (2023).
Zhang, C., Vinogradova, E. V., Spokoyny, A. M., Buchwald, S. L. & Pentelute, B. L. Arylation chemistry for bioconjugation. Angew. Chem. Int. Ed. 58, 4810–4839 (2019).
Carrico, I. S. Chemoselective modification of proteins: hitting the target. Chem. Soc. Rev. 37, 1423–1431 (2008).
Xue, L., Karpenko, I. A., Hiblot, J. & Johnsson, K. Imaging and manipulating proteins in live cells through covalent labelling. Nat. Chem. Biol. 11, 917–923 (2015).
Acknowledgements
The financial support received from the Science and Engineering Research Board (CRG/2022/004197) is gratefully acknowledged. The authors are thankful for the financial support from Bristol Myers Squibb. Financial support received from the University Grants Commission–India (fellowship to S.M.), Prime Minister’s Research Fellows – India (Ministry of Human Resource Development) (fellowship to P.G. and S.G.) and Indian Institute of Technology Bombay (fellowship to S.R., D.R. and V.S.) are gratefully acknowledged. G.K.L. acknowledges support from the J.C. Bose fellowship.
Author information
Authors and Affiliations
Contributions
S.M. and D.M. conceived the concept. All authors designed, performed and analysed the experiments. D.M. supervised the experimental work. S.M. and D.M. prepared the paper.
Corresponding author
Ethics declarations
Competing interests
A patent application (application number 202321010040) has been filed by the Indian Institute of Technology Bombay based on the work described in this paper. The authors declare no other competing financial interests.
Peer review
Peer review information
Nature Catalysis thanks Krishnamoorthy Muralirajan, Da-Gang Yu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Tables 1–19, Figs. 1–9 and references.
Supplementary Data 1
CheckCIF data of all crystals reported in the article.
Supplementary Data 2
Crystallographic data for L80; CCDC reference 2220229.
Supplementary Data 3
Crystallographic data for complex E; CCDC reference 2220165.
Supplementary Data 4
Crystallographic data for complex F; CCDC reference 2286772.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maiti, S., Ghosh, P., Raja, D. et al. Light-induced Pd catalyst enables C(sp2)–C(sp2) cross-electrophile coupling bypassing the demand for transmetalation. Nat Catal 7, 285–294 (2024). https://doi.org/10.1038/s41929-024-01109-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-024-01109-4