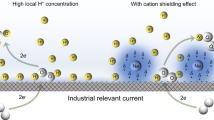
Abstract
Earth-abundant, acid-stable catalysts for the oxygen evolution reaction are essential for terawatt-scale hydrogen production using proton exchange membrane (PEM) electrolysers. Here we report that optimizing the lattice oxygen structure of manganese oxide allows it to sustain the oxygen evolution reaction for over one month at 1,000 mA cm−2 in 1 M H2SO4. The lifetime enhancement was achieved by substituting pyramidal oxygen with planar oxygen, which has a stronger Mn–O bond and thus suppresses the dissolution of manganese ions. Calculations show that the lattice oxygen dissolution is the bottleneck of deactivation, and this process is less favourable by over 0.2 eV on planar oxygen compared with pyramidal oxygen. Our material shows excellent performance even in a PEM electrolyser, reaching 2,000 mA cm−2 at 2 V with durability exceeding 1,000 h at 200 mA cm−2. This study expands the potential of Earth-abundant catalysts for PEM electrolysis, which may mitigate the reliance on iridium.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon request. The atomic coordinates of the computational models are given in Supplementary Data 1.
References
Chu, S. & Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 488, 294–303 (2012).
Lagadec, M. F. & Grimaud, A. Water electrolysers with closed and open electrochemical systems. Nat. Mater. 19, 1140–1150 (2020).
Carmo, M., Fritz, D. L., Mergel, J. & Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrog. Energy 38, 4901–4934 (2013).
Kibsgaard, J. & Chorkendorff, I. Considerations for the scaling-up of water splitting catalysts. Nat. Energy 4, 430–433 (2019).
Hubert, M. A., King, L. A. & Jaramillo, T. F. Evaluating the case for reduced precious metal catalysts in proton exchange membrane electrolyzers. ACS Energy Lett. 7, 17–23 (2021).
Scott, S. B. et al. The low overpotential regime of acidic water oxidation part II: trends in metal and oxygen stability numbers. Energy Environ. Sci. 15, 1988–2001 (2022).
Geiger, S. et al. The stability number as a metric for electrocatalyst stability benchmarking. Nat. Catal. 1, 508–515 (2018).
Huynh, M., Ozel, T., Liu, C., Lau, E. C. & Nocera, D. G. Design of template-stabilized active and Earth-abundant oxygen evolution catalysts in acid. Chem. Sci. 8, 4779–4794 (2017).
Ouimet, R. J. et al. The role of electrocatalysts in the development of gigawatt-scale PEM electrolyzers. ACS Catal. 12, 6159–6171 (2022).
Frydendal, R., Paoli, E. A., Chorkendorff, I., Rossmeisl, J. & Stephens, I. E. L. Toward an active and stable catalyst for oxygen evolution in acidic media: Ti-stabilized MnO2. Adv. Energy Mater. 5, 1500991 (2015).
Moreno-Hernandez, I. A. et al. Crystalline nickel manganese antimonate as a stable water-oxidation catalyst in aqueous 1.0 M H2SO4. Energy Environ. Sci. 10, 2103–2108 (2017).
Huynh, M., Bediako, D. K. & Nocera, D. G. A functionally stable manganese oxide oxygen evolution catalyst in acid. J. Am. Chem. Soc. 136, 6002–6010 (2014).
Yu, J. et al. Sustainable oxygen evolution electrocatalysis in aqueous 1 M H2SO4 with Earth abundant nanostructured Co3O4. Nat. Commun. 13, 4341 (2022).
Zhao, Z. et al. Tailoring manganese oxide nanoplates enhances oxygen evolution catalysis in acid. J. Catal. 405, 265–272 (2022).
Huang, J. et al. Modifying redox properties and local bonding of Co3O4 by CeO2 enhances oxygen evolution catalysis in acid. Nat. Commun. 12, 3036 (2021).
Zhou, L. et al. Rutile alloys in the Mn–Sb–O system stabilize Mn3+ to enable oxygen evolution in strong acid. ACS Catal. 8, 10938–10948 (2018).
Li, A. et al. Stable potential windows for long-term electrocatalysis by manganese oxides under acidic conditions. Angew. Chem. Int. Ed. 58, 5054–5058 (2019).
Hayashi, T., Bonnet-Mercier, N., Yamaguchi, A., Suetsugu, K. & Nakamura, R. Electrochemical characterization of manganese oxides as a water oxidation catalyst in proton exchange membrane electrolysers. R. Soc. Open Sci. 6, 190122 (2019).
Pan, S. et al. Efficient and stable noble-metal-free catalyst for acidic water oxidation. Nat. Commun. 13, 2294 (2022).
Liu, D.-J. (Invited) On the structural and mechanistic studies of PGM-free OER catalysts for PEM electrolyzer. ECS Meet. Abstr. MA2022-01, 1755–1755 (2022).
Rodríguez-García, B. et al. Cobalt hexacyanoferrate supported on Sb-doped SnO2 as a non-noble catalyst for oxygen evolution in acidic medium. Sustain. Energy Fuels 2, 589–597 (2018).
Li, A. et al. Enhancing the stability of cobalt spinel oxide towards sustainable oxygen evolution in acid. Nat. Catal. 5, 109–118 (2022).
Chatti, M. et al. Intrinsically stable in situ generated electrocatalyst for long-term oxidation of acidic water at up to 80 °C. Nat. Catal. 2, 457–465 (2019).
Yang, X. et al. Highly acid-durable carbon coated Co3O4 nanoarrays as efficient oxygen evolution electrocatalysts. Nano Energy 25, 42–50 (2016).
Chong, L. et al. La- and Mn-doped cobalt spinel oxygen evolution catalyst for proton exchange membrane electrolysis. Science 380, 609–616 (2023).
Wang, Z., Zheng, Y.-R., Chorkendorff, I. & Nørskov, J. K. Acid-stable oxides for oxygen electrocatalysis. ACS Energy Lett. 5, 2905–2908 (2020).
Back, S., Tran, K. & Ulissi, Z. W. Discovery of acid-stable oxygen evolution catalysts: high-throughput computational screening of equimolar bimetallic oxides. ACS Appl. Mater. Interfaces 12, 38256–38265 (2020).
Chabre, Y. & Pannetier, J. Structure and electrochemical properties of the proton/γ-MnO2 system. Prog. Solid St. Chem. 23, 1–130 (1995).
Zhu, H. et al. Bridging structural inhomogeneity to functionality: pair distribution function methods for functional materials development. Adv. Sci. 8, 2003534 (2021).
Egami, T. & Billinge, S. J. L. Underneath the Bragg Peaks: Structural Analysis of Complex Materials. (Pergamon, 2003).
Juhas, P., Davis, T., Farrow, C. L. & Billinge, S. J. L. PDFgetX3: a rapid and highly automatable program for processing powder diffraction data into total scattering pair distribution functions. J. Appl. Crystallogr. 46, 560–566 (2013).
Galliez, K. et al. Modelling and quantification of intergrowth in γ-MnO2 by laboratory pair distribution function analysis. J. Appl. Crystallogr. 47, 552–560 (2014).
Magnard, N. P. L., Anker, A. S., Aalling-Frederiksen, O., Kirsch, A. & Jensen, K. M. Ø. Characterization of intergrowth in metal oxide materials using structure-mining: the case of γ-MnO2. Dalton Trans. 51, 17150–17161 (2022).
Hatakeyama, T., Okamoto, N. L. & Ichitsubo, T. Thermal stability of MnO2 polymorphs. J. Solid State Chem. 305, 122683 (2022).
Petit, F., Durr, J., Lenglet, M. & Hannoyer, B. Thermal behavior of gamma manganese dioxide. Mat. Res. Bull. 28, 959–966 (1993).
Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions (National Association of Corrosion Engineers, 1974).
Bard, A. J., Faulkner, L. R. Electrochemical Methods: Fundamentals and Applications (John Wiley, 2001).
Ayers, K. et al. Perspectives on low-temperature electrolysis and potential for renewable hydrogen at scale. Annu. Rev. Chem. Biomol. Eng. 10, 219–239 (2019).
Kato, K., Tanaka, Y., Yamauchi, M., Ohara, K. & Hatsui, T. A statistical approach to correct X-ray response non-uniformity in microstrip detectors for high-accuracy and high-resolution total-scattering measurements. J. Synchrotron Radiat. 26, 762–773 (2019).
Kato, K. et al. The RIKEN Materials Science Beamline at SPring-8: towards visualization of electrostatic interaction. In Proc. 10th International Conference on Synchrotron Radiation Instrumentation, SRI 2009, Melbourne, Victoria, 875–878 (2010); https://doi.org/10.1063/1.3463354
Kato, K. & Tanaka, H. Visualizing charge densities and electrostatic potentials in materials by synchrotron X-ray powder diffraction. Adv. Phys. X 1, 55–80 (2016).
Farrow, C. L. et al. PDFfit2 and PDFgui: computer programs for studying nanostructure in crystals. J. Condens. Matter Phys. 19, 335219 (2007).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Hammer, B., Hansen, L. B. & Nørskov, J. K. Improved adsorption energetics within density-functional theory using revised Perdew–Burke–Ernzerhof functionals. Phys. Rev. B 59, 7413–7421 (1999).
Caltagirone, S. & Massingill, J. Developing γ-MnO2 models for XRD analysis. ECS Trans. 11, 29–35 (2008).
Yuan, Y. et al. Revealing the atomic structures of exposed lateral surfaces for polymorphic manganese dioxide nanowires. Small Struct. 2, 2000091 (2021).
Chan, K. & Nørskov, J. K. Electrochemical barriers made simple. J. Phys. Chem. Lett. 6, 2663–2668 (2015).
Liu, X. et al. pH effects on the electrochemical reduction of CO(2) towards C2 products on stepped copper. Nat. Commun. 10, 32 (2019).
Shi, Y. et al. Unveiling hydrocerussite as an electrochemically stable active phase for efficient carbon dioxide electroreduction to formate. Nat. Commun. 11, 3415 (2020).
Acknowledgements
The synchrotron radiation experiments were performed at BL14B2 (XAFS) and BL44B2 (SR-PXRD) of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (proposal numbers 2021A1664, 2022B1667 and 2022A1045) and RIKEN (proposal numbers 20210064, 20220057 and 20230043). We thank H. Ofuchi (JASRI) for the XAFS experiments, and K. Kato (RIKEN) and K. Shigeta (Nippon Gijutsu Center) for the SR-PXRD and total scattering experiments. We thank T. Wakashima (RIKEN) for his assistance with creating the graphical abstract. This work was partially supported by the New Energy and Industrial Technology Development Organization (NEDO, JPNP14021). R.N. acknowledges JSPS Grant-in-Aid for Scientific Research (22H00339). J.X. acknowledges financial supports from the National Key Research and Development Program of China (2021YFA1500702), the National Natural Science Foundation of China (22172156 and 22321002), the AI S&T Program of Yulin Branch, Dalian National Laboratory for Clean Energy, CAS (DNL-YLA202205, J.X.) and DNL Cooperation Fund, CAS (DNL202003).
Author information
Authors and Affiliations
Contributions
S.K., A.L. and R.N. conceived and designed the experiments. S.K., A.L., K.A., K.F. and Q.J. performed the experiments. J.L. and J.X. performed the DFT calculations. S.K., A.L., H.O., K.A., D.H. and R.N. analysed the data. S.K., A.L., J.L., H.O., J.X. and R.N. co-wrote the paper. All the authors discussed the results and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Bin Dong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–50, Tables 1–13, Notes 1–4 and References.
Supplementary Data 1
The atomic coordinates of the computational models.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kong, S., Li, A., Long, J. et al. Acid-stable manganese oxides for proton exchange membrane water electrolysis. Nat Catal 7, 252–261 (2024). https://doi.org/10.1038/s41929-023-01091-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01091-3
This article is cited by
-
Durable MnO2 electrocatalysts by stronger Mn–O bonds
Nature Catalysis (2024)