Abstract
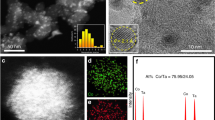
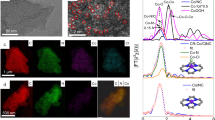
Metal-nitrogen-doped carbon (M-N-C) catalysts are promising platinum group metals alternatives for fuel cell cathodes; however, their randomly formed, unpredictable coordinations complicate any structure–property investigation and lead to low active site density. Here we achieve dense and well-defined cobalt sites on the surfaces of Co(CN)3 microcrystals, which are identified by single-crystal X-ray diffraction and X-ray absorption spectroscopy. In situ infrared spectroscopy and density functional theory calculations elucidate that the high oxygen reduction reaction performance (half-wave potential is 0.90 V versus RHE) of the cubic Co(CN)3 is due to its tailored coordination environment that optimizes *OH desorption. The cyanide linkage with minimized spacing between cobalt atoms maximizes active sites’ spatial density, which boosts the anion-exchange membrane fuel cell peak power density to 1.67 W cm−2. The microcrystals with well-defined coordination structures can be used as Co-N-C analogues for their structure–property relationship investigations.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The crystallographic information file obtained directly from SCXRD has been deposited in the CCDC with reference number of 2291782. Other datasets generated during and/or analysed during the current study are available from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Steele, B. C. H. & Heinzel, A. Materials for fuel-cell technologies. Nature 414, 345–352 (2001).
Wang, C.-Y. Fundamental models for fuel cell engineering. Chem. Rev. 104, 4727–4766 (2004).
Zhu, C., Li, H., Fu, S., Du, D. & Lin, Y. Highly efficient nonprecious metal catalysts towards oxygen reduction reaction based on three-dimensional porous carbon nanostructures. Chem. Soc. Rev. 45, 517–531 (2016).
Zhang, J. et al. Recent insights on catalyst layers for anion exchange membrane fuel cells. Adv. Sci. 8, 2100284 (2021).
Yan, L. et al. Atomically precise electrocatalysts for oxygen reduction reaction. Chem 9, 280–432 (2023).
Sui, S. et al. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 5, 1808–1825 (2017).
Wu, J. & Yang, H. Platinum-based oxygen reduction electrocatalysts. Acc. Chem. Res. 46, 1848–1857 (2013).
Stamenkovic, V. R. et al. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 315, 493–497 (2007).
Hossen, M. M. et al. State-of-the-art and developmental trends in platinum group metal-free cathode catalyst for anion exchange membrane fuel cell (AEMFC). Appl. Catal. B 325, 121733 (2023).
Ji, S. et al. Chemical synthesis of single atomic site catalysts. Chem. Rev. 120, 11900–11955 (2020).
Adabi, H. et al. High-performing commercial Fe–N–C cathode electrocatalyst for anion-exchange membrane fuel cells. Nat. Energy 6, 834–843 (2021).
He, Q. et al. Polymer-coating-induced synthesis of FeNx enriched carbon nanotubes as cathode that exceeds 1.0 W cm−2 peak power in both proton and anion exchange membrane fuel cells. J. Power Sources 489, 229499 (2021).
Palaniselvam, T., Kashyap, V., Bhange, S. N., Baek, J.-B. & Kurungot, S. Nanoporous graphene enriched with Fe/Co-N active sites as a promising oxygen reduction electrocatalyst for anion exchange membrane fuel cells. Adv. Funct. Mater. 26, 2150–2162 (2016).
Zhang, H. et al. High-performance fuel cell cathodes exclusively containing atomically dispersed iron active sites. Energy Environ. Sci. 12, 2548–2558 (2019).
Woo, J. et al. Promoting oxygen reduction reaction activity of Fe-N/C electrocatalysts by silica-coating-mediated synthesis for anion-exchange membrane fuel cells. Chem. Mater. 30, 6684–6701 (2018).
Liu, J. et al. High performance platinum single atom electrocatalyst for oxygen reduction reaction. Nat. Commun. 8, 15938 (2017).
Peng, L. et al. Mesopore-rich Fe–N–C catalyst with FeN4–O–NC single-atom sites delivers remarkable oxygen reduction reaction performance in alkaline media. Adv. Mater. 34, e2202544 (2022).
Saladino, R., Crestini, C., Ciciriello, F., Costanzo, G. & Di Mauro, E. Formamide chemistry and the origin of informational polymers. Chem. Biodivers. 4, 694–720 (2007).
Nguyen, V. S. et al. Theoretical study of formamide decomposition pathways. J. Phys. Chem. A 115, 841–851 (2011).
Gahlaut, A. & Paranjothy, M. Unimolecular decomposition of formamide via direct chemical dynamics simulations. Phys. Chem. Chem. Phys. 20, 8498–8505 (2018).
Yeo, B.-E., Cho, Y.-S. & Huh, Y.-D. Evolution of the morphology of Cu2O microcrystals: cube to 50-facet polyhedron through beveled cube and rhombicuboctahedron. CrystEngComm 19, 1627–1632 (2017).
Robin, M. B. The color and electronic configurations of Prussian blue. Inorg. Chem. 1, 337–342 (1962).
Kaye, S. S. & Long, J. R. Hydrogen storage in the dehydrated Prussian blue analogues M3[Co(CN)6]2 (M = Mn, Fe, Co, Ni, Cu, Zn). J. Am. Chem. Soc. 127, 6506–6507 (2005).
Wessells, C. D., Huggins, R. A. & Cui, Y. Copper hexacyanoferrate battery electrodes with long cycle life and high power. Nat. Commun. 2, 550 (2011).
Vannerberg, N. G. The ESCA-spectra of sodium and potassium cyanide and of the sodium and potassium salts of the hexacyanometallates of the first transition metal series. Chem. Scr. 9, 122–126 (1976).
Zakaria, M. B. & Chikyow, T. Recent advances in Prussian blue and Prussian blue analogues: synthesis and thermal treatments. Coord. Chem. Rev. 352, 328–345 (2017).
Lee, H. W. et al. Manganese hexacyanomanganate open framework as a high-capacity positive electrode material for sodium-ion batteries. Nat. Commun. 5, 5280 (2014).
Zhao, K.-M. et al. Insight into the mechanism of axial ligands regulating the catalytic activity of Fe-N4 sites for oxygen reduction reaction. Adv. Energy Mater. 12, 2103588 (2022).
Liu, X., Liu, Y., Yang, W., Feng, X. & Wang, B. Controlled axial coordination modification for transition metal single atom electrocatalyst. Chem. Eur. J. 28, e202201471 (2022).
Zhao, T. et al. Assisting atomic dispersion of Fe in N-doped carbon by aerosil for high-efficiency oxygen reduction. ACS Appl. Mater. Interfaces 12, 25832–25842 (2020).
Li, L. et al. Optimizing microenvironment of asymmetric N,S-coordinated single-atom Fe via axial fifth coordination toward efficient oxygen electroreduction. Small 18, 2105387 (2022).
Holst-Olesen, K., Reda, M., Hansen, H. A., Vegge, T. & Arenz, M. Enhanced oxygen reduction activity by selective anion adsorption on non-precious-metal catalysts. ACS Catal. 8, 7104–7112 (2018).
Holst-Olesen, K., Silvioli, L., Rossmeisl, J. & Arenz, M. Enhanced oxygen reduction reaction on Fe/N/C catalyst in acetate buffer electrolyte. ACS Catal. 9, 3082–3089 (2019).
Wei, C. et al. Recommended practices and benchmark activity for hydrogen and oxygen electrocatalysis in water splitting and fuel cells. Adv. Mater. 31, 1806296 (2019).
Lazaridis, T., Stuhmeier, B. M., Gasteiger, H. A. & El-Sayed, H. A. Capabilities and limitations of rotating disk electrodes versus membrane electrode assemblies in the investigation of electrocatalysts. Nat. Catal. 5, 363–373 (2022).
Liao, X. et al. Density functional theory for electrocatalysis. Energy Environ. Mater. 5, 157–185 (2021).
Liu, C. et al. Intrinsic activity of metal centers in metal–nitrogen–carbon single-atom catalysts for hydrogen peroxide synthesis. J. Am. Chem. Soc. 142, 21861–21871 (2020).
Tang, C. et al. Tailoring acidic oxygen reduction selectivity on single-atom catalysts via modification of first and second coordination spheres. J. Am. Chem. Soc. 143, 7819–7827 (2021).
Zhao, L. et al. Cascade anchoring strategy for general mass production of high-loading single-atomic metal-nitrogen catalysts. Nat. Commun. 10, 1278 (2019).
Mathew, K., Sundararaman, R., Letchworth-Weaver, K., Arias, T. A. & Hennig, R. G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 140, 084106 (2014).
Fishman, M., Zhuang, H. L., Mathew, K., Dirschka, W. & Hennig, R. G. Accuracy of exchange-correlation functionals and effect of solvation on the surface energy of copper. Phys. Rev. B 87, 245402 (2013).
Dong, J. C. et al. Direct in situ Raman spectroscopic evidence of oxygen reduction reaction intermediates at high-index Pt(hkl) surfaces. J. Am. Chem. Soc. 142, 715–719 (2020).
Dong, J. C. et al. In situ Raman spectroscopic evidence for oxygen reduction reaction intermediates at platinum single-crystal surfaces. Nat. Energy 4, 60–67 (2019).
Pan, Y. et al. Construction of N, P Co-doped carbon frames anchored with Fe single atoms and Fe2P nanoparticles as a robust coupling catalyst for electrocatalytic oxygen reduction. Adv. Mater. 34, 2203621 (2022).
Tanaka, H. et al. Infrared reflection absorption spectroscopy of OH adsorption on the low index planes of Pt. Electrocatalysis 6, 295–299 (2015).
Mawhinney, D. B., Glass, J. A. & Yates, J. T. FTIR study of the oxidation of porous silicon. J. Phys. Chem. B 101, 1202–1206 (1997).
Enders, D., Nagao, T., Pucci, A., Nakayama, T. & Aono, M. Surface-enhanced ATR-IR spectroscopy with interface-grown plasmonic gold-island films near the percolation threshold. Phys. Chem. Chem. Phys. 13, 4935–4941 (2011).
Yao, Y., Zhu, S., Wang, H., Li, H. & Shao, M. A spectroscopic study on the nitrogen electrochemical reduction reaction on gold and platinum surfaces. J. Am. Chem. Soc. 140, 1496–1501 (2018).
Kulkarni, A., Siahrostami, S., Patel, A. & Norskov, J. K. Understanding catalytic activity trends in the oxygen reduction reaction. Chem. Rev. 118, 2302–2312 (2018).
Nørskov, J. K., Studt, F., Abild-Pedersen, F. & Bligaard, T. in Fundamental Concepts in Heterogeneous Catalysis Ch. 8, 114–137 (John Wiley & Sons, 2014).
Jia, Y. et al. Atomically dispersed Fe-N4 modified with precisely located S for highly efficient oxygen reduction. Nanomicro Lett. 12, 116 (2020).
Mun, Y. et al. Versatile strategy for tuning ORR activity of a single Fe-N4 site by controlling electron-withdrawing/donating properties of a carbon plane. J. Am. Chem. Soc. 141, 6254–6262 (2019).
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42, 339–341 (2009).
Sheldrick, G. Crystal structure refinement with SHELXL. Acta Cryst. C71, 3–8 (2015).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 12, 537–541 (2005).
Bunau, O. & Joly, Y. Self-consistent aspects of X-ray absorption calculations. J. Phys. Condens. Matter 21, 345501 (2009).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Kresse, G. F. Jürgen efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Lin, C. Y., Zhang, L., Zhao, Z. & Xia, Z. Design principles for covalent organic frameworks as efficient electrocatalysts in clean energy conversion and green oxidizer production. Adv. Mater. 29, 1606635 (2017).
Gong, L. L. et al. Catalytic mechanisms and design principles for single-atom catalysts in highly efficient CO2 conversion. Adv. Energy Mater. 9, 1902625 (2019).
Wang, V., Xu, N., Liu, J.-C., Tang, G. & Geng, W.-T. VASPKIT: a user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 267, 108033 (2021).
Acknowledgements
X.S. acknowledges financial support from the National Key Research and Development Program of China (2018YFA0702002, 2022YFA1504000), the Beijing Natural Science Foundation (Z210016) and the National Natural Science Foundation of China (21935001), Z.Z. acknowledges financial support from the National Key Research and Development Program of China (2019YFA0210300), S.T. acknowledges financial support from the National Natural Science Foundation of China (22101015) and the Fundamental Research Funds of Beijing University of Chemical Technology (buctrc202107) and J.D. acknowledges financial support from the Youth Innovation Promotion Association of the Chinese Academy of Sciences (Y2022006).
Author information
Authors and Affiliations
Contributions
K.S. designed and performed the synthetic and electrochemical experiments, characterized the catalyst and analysed the data. J.D. completed the XAFS characterization and corresponding data analysis. H.S. conducted the DFT calculations. X.W. assisted with the AEMFC testing. J.F. assisted with the in situ ATR-SEIRAS tests and data analysis. S.T., Z.Z. and X.S. supervised the execution of the overall project. The results of the manuscript were discussed by all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Yaqiong Su and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–29, Tables 1–5 and Notes 1 and 2.
Crystallographic Data 1
Crystallographic Information File obtained directly from SCXRD with CCDC no. 2291782.
Crystallographic Data 2
Crystallographic Information File of Co(CN)3 refined by XAS.
Crystallographic Data 3
The optimised strctures using for calcuating the density of states of each crystal plane and partial density of states of cobalt in each crystal plane of Co(CN)3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, K., Dong, J., Sun, H. et al. Co(CN)3 catalysts with well-defined coordination structure for the oxygen reduction reaction. Nat Catal 6, 1164–1173 (2023). https://doi.org/10.1038/s41929-023-01047-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01047-7