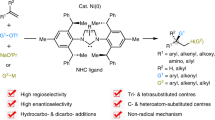
Abstract
Metal-catalysed cross-coupling has played a central role in modern chemical synthesis for decades. Unactivated alkenes, including light olefins, are manufactured on enormous scale in the petroleum industry and represent ideal starting materials for the preparation of pharmaceuticals, agrochemicals and materials. However, enantioselective cross-coupling of unactivated alkenes remains a challenging and long-standing goal. Here we report a highly enantio- and regioselective three-component cross-coupling of unactivated alkenes with aryl (or alkenyl) triflates and organometallics (or reductant) to construct diverse C sp3 stereocentres by nickel catalysis. Specifically, selective carbonickelation and in situ trapping with nucleophiles enable efficient hydrofunctionalization and dicarbofunctionalization of unactivated alkenes in a directing group-free manner. Nickel catalysts bearing bulky C2-symmetric chiral N-heterocyclic carbene ligands were crucial for attaining high reactivity and selectivity. This strategy offers a general, modular and divergent platform for rapidly upgrading feedstock alkenes to various value-added molecules and is expected to inspire the development of other challenging enantioselective alkene cross-couplings.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the article and its Supplementary Information. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number 2223535 (7d). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures.
References
Meijere, A. D., Bräse, S. & Oestreich, M. (eds) Metal-Catalyzed Cross-Coupling Reactions and More (Wiley-VCH, 2014).
Jana, R., Pathak, T. P. & Sigman, M. S. Advances in transition metal (Pd,Ni,Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners. Chem. Rev. 111, 1417–1492 (2011).
Johansson Seechurn, C. C., Kitching, M. O., Colacot, T. J. & Snieckus, V. Palladium-catalyzed cross-coupling: a historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 51, 5062–5085 (2012).
Cherney, A. H., Kadunce, N. T. & Reisman, S. E. Enantioselective and enantiospecific transition-metal-catalyzed cross-coupling reactions of organometallic reagents to construct C–C bonds. Chem. Rev. 115, 9587–9652 (2015).
Zhao, S. et al. Enantiodivergent Pd-catalyzed C–C bond formation enabled through ligand parameterization. Science 362, 670–674 (2018).
Bonet, A., Odachowski, M., Leonori, D., Essafi, S. & Aggarwal, V. K. Enantiospecific sp2–sp3 coupling of secondary and tertiary boronic esters. Nat. Chem. 6, 584–589 (2014).
Choi, J. & Fu, G. C. Transition-metal-catalyzed alkyl–alkyl bond formation: another dimension in cross-coupling chemistry. Science 356, 7230–7237 (2017).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Geilen, F. M. A., Stochniol, G., Peitz, S. & Schulte-Koerne, E. in Ullmann’s Encyclopedia of Industrial Chemistry (eds Obenaus, F. et al.) 1–13 (Wiley, 2014).
Grela, K. Olefin Metathesis: Theory and Practice (Wiley, 2014).
Davarnejad, R. Alkenes–Recent Advances, New Perspectives and Applications (Intechopen, 2021).
Qi, X. & Diao, T. Nickel-catalyzed dicarbofunctionalization of alkenes. ACS Catal. 10, 8542–8556 (2020).
Zhu, S., Zhao, X., Li, H. & Chu, L. Catalytic three-component dicarbofunctionalization reactions involving radical capture by nickel. Chem. Soc. Rev. 50, 10836–10856 (2021).
Sun, X. Y., Yao, B. Y., Xuan, B., Xiao, L. J. & Zhou, Q.-L. Recent advances in nickel-catalyzed asymmetric hydrofunctionalization of alkenes. Chem. Catal. 2, 3140–3162 (2022).
Zhang, L. et al. Catalytic conjunctive cross-coupling enabled by metal-induced metallate rearrangement. Science 351, 70–74 (2016).
Wu, L. et al. Asymmetric Cu-catalyzed intermolecular trifluoromethylarylation of styrenes: enantioselective arylation of benzylic radicals. J. Am. Chem. Soc. 139, 2904–2907 (2017).
Wei, X., Shu, W., García-Domínguez, A., Merino & Nevado, E. C. Asymmetric Ni-catalyzed radical-relayed reductive coupling. J. Am. Chem. Soc. 142, 13515–13522 (2020).
Dong, X.-Y. et al. Copper-catalyzed asymmetric radical 1,2-carboalkynylation of alkenes with alkyl halides and terminal alkynes. J. Am. Chem. Soc. 142, 9501–9509 (2020).
Xi, Y. et al. Catalytic asymmetric diarylation of internal acyclic styrenes and enamides. J. Am. Chem. Soc. 144, 8389–8398 (2022).
Liu, C. F. et al. Synthesis of tri-and tetrasubstituted stereocentres by nickel-catalysed enantioselective olefin cross-couplings. Nat. Catal. 5, 934–942 (2022).
Wang, Z., Yin, H. & Fu, G. C. Catalytic enantioconvergent coupling of secondary and tertiary electrophiles with olefins. Nature 563, 379–383 (2018).
Mei, T. S., Patel, H. H. & Sigman, M. S. Enantioselective construction of remote quaternary stereocentres. Nature 508, 340–344 (2014).
Mlynarski, S., Schuster, C. & Morken, J. Asymmetric synthesis from terminal alkenes by cascades of diboration and cross-coupling. Nature 505, 386–390 (2014).
Wang, W., Ding, C. & Yin, G. Catalyst-controlled enantioselective 1,1-arylboration of unactivated olefins. Nat. Catal. 3, 951–958 (2020).
Stradiotto, M. & Lundgren, R. J. Ligand Design in Metal Chemistry: Reactivity and Catalysis (Wiley, 2016).
Giri, R. & Kc, S. Strategies toward dicarbofunctionalization of unactivated olefins by combined Heck carbometalation and cross-coupling. J. Org. Chem. 83, 3013–3022 (2018).
Coombs, J. R. & Morken, J. P. Catalytic enantioselective functionalization of unactivated terminal alkenes. Angew. Chem. Int. Ed. 55, 2636–2649 (2016).
Janssen‐Müller, D., Sahoo, B., Sun, S. Z. & Martin, R. Tackling remote sp3 C–H functionalization via Ni-catalyzed ‘chain-walking’ reactions. Isr. J. Chem. 60, 195–206 (2020).
Wang, Z. X., Bai, X. Y. & Li, B. J. Metal-catalyzed substrate-directed enantioselective functionalization of unactivated alkenes. Chin. J. Chem. 37, 1174–1180 (2019).
Wang, H. et al. Palladium-catalyzed amide-directed enantioselective hydrocarbofunctionalization of unactivated alkenes using a chiral monodentate oxazoline ligand. J. Am. Chem. Soc. 140, 3542–3546 (2018).
Apolinar, O. et al. Three-component asymmetric Ni-catalyzed 1,2-dicarbofunctionalization of unactivated alkenes via stereoselective migratory insertion. J. Am. Chem. Soc. 144, 19337–19343 (2022).
Tu, H. Y. et al. Enantioselective three-component fluoroalkylarylation of unactivated olefins through nickel-catalyzed cross-electrophile coupling. J. Am. Chem. Soc. 142, 9604–9611 (2020).
Teng, S. & Zhou, J. S. Metal-catalyzed asymmetric heteroarylation of alkenes: diverse activation mechanisms. Chem. Soc. Rev. 51, 1592–1607 (2022).
Ping, Y., Li, Y., Zhu, J. & Kong, W. Construction of quaternary stereocenters by palladium-catalyzed carbopalladation-initiated cascade reactions. Angew. Chem. Int. Ed. 58, 1562–1573 (2019).
Liu, C. F., Luo, X., Wang, H. & Koh, M. J. Catalytic regioselective olefin hydroarylation (alkenylation) by sequential carbonickelation–hydride transfer. J. Am. Chem. Soc. 143, 9498–9506 (2021).
Wang, H., Liu, C. F., Martin, R. T., Gutierrez, O. & Koh, M. J. Directing-group-free catalytic dicarbofunctionalization of unactivated alkenes. Nat. Chem. 14, 188–195 (2022).
Janssen-Müller, D., Schlepphorst, C. & Glorius, F. Privileged chiral N-heterocyclic carbene ligands for asymmetric transition-metal catalysis. Chem. Soc. Rev. 46, 4845–4854 (2017).
Wang, Z.-C., Xie, P.-P., Xu, Y., Hong, X. & Shi, S.-L. Low-temperature nickel-catalysed C−N cross-coupling via kinetic resolution enabled by a bulky and flexible chiral N-heterocyclic carbene ligand. Angew. Chem. Int. Ed. 60, 16077–16084 (2021).
Shen, D., Xu, Y. & Shi, S.-L. A bulky chiral N-heterocyclic carbene palladium catalyst enables highly enantioselective Suzuki–Miyaura cross-coupling reactions for the synthesis of biaryl atropisomers. J. Am. Chem. Soc. 141, 14938–14945 (2019).
Ruan, L.-X., Sun, B., Liu, J.-M. & Shi, S.-L. Dynamic kinetic asymmetric arylation and alkenylation of ketones. Science 379, 662–670 (2023).
Cai, Y. et al. Copper-catalyzed enantioselective Markovnikov protoboration of α-olefins enabled by a buttressed N-heterocyclic carbene ligand. Angew. Chem. Int. Ed. 57, 1376–1380 (2018).
Zhang, W.-B., Yang, X.-T., Ma, J.-B., Su, Z.-M. & Shi, S.-L. Regio-and enantioselective C–H cyclization of pyridines with alkenes enabled by a nickel/N-heterocyclic carbene catalysis. J. Am. Chem. Soc. 141, 5628–5634 (2019).
Ma, J.-B., Zhao, X., Zhang, D. & Shi, S.-L. Enantio- and regioselective Ni-catalyzed para-C–H alkylation of pyridines with styrenes via intermolecular hydroarylation. J. Am. Chem. Soc. 144, 13643–13651 (2022).
Beletskaya, I. P. & Cheprakov, A. V. The Heck reaction as a sharpening stone of palladium catalysis. Chem. Rev. 100, 3009–3066 (2000).
Song, G., Wylie, N. O. W. & Hou, Z. Enantioselective C–H bond addition of pyridines to alkenes catalyzed by chiral half-sandwich rare-earth complexes. J. Am. Chem. Soc. 136, 12209–12212 (2014).
Sun, X., Lin, E. Z. & Li, B. J. Iridium-catalyzed branch-selective and enantioselective hydroalkenylation of α-olefins through C–H cleavage of enamides. J. Am. Chem. Soc. 144, 17351–17358 (2022).
Gao, P., Chen, L. A. & Brown, M. K. Nickel-catalyzed stereoselective diarylation of alkenylarenes. J. Am. Chem. Soc. 140, 10653–10657 (2018).
Meroueh, S. O. Compounds and methods for treating cancer by inhibiting the urokinase receptor. US patent 20150051247A1 (2015).
Stoit, A. et al. Preparation of spirocyclic amine derivatives for use as S1P modulators. WO patent 2012004378A1 (2012).
Chinta, R. R. et al. Total synthesis of S-(+)-curcuphenol, S-(+)-curcuquinone and S-(+)-curcuhydroquinone. Asian J. Org. Chem. 28, 771–778 (2016).
Sasata, Y., Yanai, M. & Yamamoto, S. Polymerizable liquid-crystal compound having optically active 1-alkylethylene or 1-oxo-2-alkylethylene bonding group for display device. JP patent 2005023019A (2005).
Acknowledgements
Financial support was provided by the National Key R&D Program of China (2022YFA1503702 and 2021YFF0701600), the National Natural Science Foundation of China (22325110, 92256303, 22171280 and 21821002), the Program of Shanghai Academic Research Leader (22XD1424900), the CAS Youth Interdisciplinary Team (JCTD-2021-11), the Ningbo Natural Science Foundation (2022J017, S.-L.S.) and the Ministry of Education of Singapore Academic Research Fund Tier 2: A-8000034-00-00 (M.J.K.).
Author information
Authors and Affiliations
Contributions
Z.-C.W., X.L., J.-W.Z. and C.-F.L. developed the catalytic method and conducted mechanistic studies. S.-L.S. and M.J.K. conceived the project and directed the investigations. S.-L.S. and Z.-C.W. wrote the paper with input from the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Naoto Chatani and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–7, Methods, NMR spectra, HPLC traces and References.
Supplementary Data 1
Cif file of crystal structure for compound 7d.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, ZC., Luo, X., Zhang, JW. et al. Enantioselective C–C cross-coupling of unactivated alkenes. Nat Catal 6, 1087–1097 (2023). https://doi.org/10.1038/s41929-023-01037-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01037-9