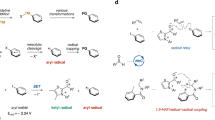
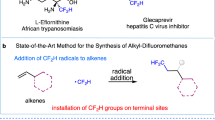
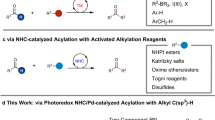
Abstract
Radical reactions play an important role in modern organic synthetic chemistry. The generation of carbon radicals from the homolytic cleavage of carbon–metal bonds has been widely studied as a fundamental step in organometallic chemistry. However, the implementation of this phenomenon in catalysed cascade reactions, that is, an organometallic-radical relay, is a highly challenging undertaking due to transient radicals generated from these thermodynamically disfavoured endergonic processes. Here we disclose a set of catalytic alkene 1,1-difunctionalization reactions conceptually based on an organometallic-radical relay. This strategy enables a diversity of sp3/sp2 fragments and aryl groups to be simultaneously introduced to the same carbon of both terminal and internal alkenes with outstanding chemo- and regioselectivity. This study provides a concept for the generation of functionalized benzylic radicals, which are difficult to access by classical protocols, from non-radical feedstock alkenes and arylboronic acids.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All information relating to optimization studies, experimental procedures, mechanistic studies, DFT calculations, NMR spectra and high-resolution mass spectrometry are available in the Supplementary Information. All other data are available from the corresponding authors upon request.
References
Plesniak, M. P., Huang, H.-M. & Procter, D. J. Radical cascade reactions triggered by single electron transfer. Nat. Rev. Chem. 1, 0077 (2017).
Jasperse, C. P., Curran, D. P. & Fevig, T. L. Radical reactions in natural product synthesis. Chem. Rev. 91, 1237–1286 (1991).
Yan, M., Lo, J. C., Edwards, J. T. & Baran, P. S. Radicals: reactive intermediates with translational potential. J. Am. Chem. Soc. 138, 12692–12714 (2016).
Pitre, S. P., Weires, N. A. & Overman, L. E. Forging C(sp3)–C(sp3) bonds with carbon-centered radicals in the synthesis of complex molecules. J. Am. Chem. Soc. 141, 2800–2813 (2019).
Hammerich, O. & Speiser, B. Organic Electrochemistry, 5th edn (CRC Press, 2015).
Stephenson, C. R. J., Yoon, T. P. & MacMillan, D. W. C. (eds) Visible Light Photocatalysis in Organic Chemistry (Wiley-VCH, 2018).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017).
Ravelli, D., Protti, S. & Fagnoni, M. Carbon–carbon bond forming reactions via photogenerated intermediates. Chem. Rev. 116, 9850–9913 (2016).
Chan, A. Y. et al. Metallaphotoredox: the merger of photoredox and transition metal catalysis. Chem. Rev. 122, 1485–1542 (2022).
Malapit, C. A. et al. Advances on the merger of electrochemistry and transition metal catalysis for organic synthesis. Chem. Rev. 122, 3180–3218 (2022).
Clayden, J., Greeves, N. & Warren, S. Organic Chemistry (Oxford University Press, 2012).
Morcillo, S. P. Radical-promoted C–C bond cleavage: a deconstructive approach for selective functionalization. Angew. Chem. Int. Ed. 58, 14044–14054 (2019).
Sivaguru, P., Wang, Z., Zanoni, G. & Bi, X. Cleavage of carbon–carbon bonds by radical reactions. Chem. Soc. Rev. 48, 2615–2656 (2019).
Crespi, S. & Fagnoni, M. Generation of alkyl radicals: from the tyranny of tin to the photon democracy. Chem. Rev. 120, 9790–9833 (2020).
Heinrich, M. & Gansäuer, A. Radicals in Synthesis III (Springer, 2012).
Crossley, S. W. M., Obradors, C., Martinez, R. M. & Shenvi, R. A. Mn-, Fe-, and Co-catalyzed radical hydrofunctionalizations of olefins. Chem. Rev. 116, 8912–9000 (2016).
Diccianni, J. B. & Diao, T. Mechanisms of nickel-catalyzed cross-coupling reactions. Trends Chem. 1, 830–844 (2019).
Dreos, R. et al. Synthesis, characterization, and reactivity of a new mononuclear benzyl–cobalt complex containing mixed tridentate imino-oxime and diamine ligands. Organometallics 17, 2366–2369 (1998).
MacLeod, K. C. et al. Masked radicals: iron complexes of trityl, benzophenone, and phenylacetylene. Organometallics 38, 4224–4232 (2019).
Verma, R. et al. Recent trends in photocatalytic enantioselective reactions. Top. Curr. Chem. 380, 48 (2022).
Huang, C. Y., Li, J. & Li, C. J. Photocatalytic C(sp3) radical generation via C–H, C–C, and C–X bond cleavage. Chem. Sci. 13, 5465–5504 (2022).
Gutierrez, O., Tellis, J. C., Primer, D. N., Molander, G. A. & Kozlowski, M. C. Nickel-catalyzed cross-coupling of photoredox-generated radicals: uncovering a general manifold for stereoconvergence in nickel-catalyzed cross-couplings. J. Am. Chem. Soc. 137, 4896–4899 (2015).
Zhu, J., Wang, Q. & Wang, M. Multicomponent Reactions in Organic Synthesis (Wiley-VCH, 2015).
Wilson, M. R. & Taylor, R. E. Strained alkenes in natural product synthesis. Angew. Chem. Int. Ed. 52, 4078–4087 (2013).
Vougioukalakis, G. C. & Grubbs, R. H. Ruthenium-based heterocyclic carbene-coordinated olefin metathesis catalysts. Chem. Rev. 110, 1746–1787 (2010).
Luo, M. J., Xiao, Q. & Li, J. H. Electro-/photocatalytic alkene-derived radical cation chemistry: recent advances in synthetic applications. Chem. Soc. Rev. 51, 7206–7237 (2022).
Vasseur, A., Bruffaerts, J. & Marek, I. Remote functionalization through alkene isomerization. Nat. Chem. 8, 209–219 (2016).
Li, Y., Wu, D., Cheng, H. G. & Yin, G. Difunctionalization of alkenes involving metal migration. Angew. Chem. Int. Ed. 59, 7990–8003 (2020).
Yang, S., Chen, Y. & Ding, Z. Recent progress of 1,1-difunctionalization of olefins. Org. Biomol. Chem. 18, 6983–7001 (2020).
Tasker, S. Z., Standley, E. A. & Jamison, T. F. Recent advances in homogeneous nickel catalysis. Nature 509, 299–309 (2014).
Li, Y. et al. Modular access to substituted cyclohexanes with kinetic stereocontrol. Science 376, 749–753 (2022).
Wang, W., Ding, C. & Yin, G. Catalyst-controlled enantioselective 1,1-arylboration of unactivated olefins. Nat. Catal. 3, 951–958 (2020).
Ding, C., Ren, Y., Sun, C., Long, J. & Yin, G. Regio- and stereoselective alkylboration of endocyclic olefins enabled by nickel catalysis. J. Am. Chem. Soc. 143, 20027–20034 (2021).
Petracca, R. et al. Chemoselective synthesis of N-terminal cysteinyl thioesters via β,γ-C,S thiol-Michael addition. Org. Lett. 21, 3281–3285 (2019).
Dadova, J., Galan, S. R. & Davis, B. G. Synthesis of modified proteins via functionalization of dehydroalanine. Curr. Opin. Chem. Biol. 46, 71–81 (2018).
Corpas, J., Kim-Lee, S. H., Mauleon, P., Arrayas, R. G. & Carretero, J. C. Beyond classical sulfone chemistry: metal- and photocatalytic approaches for C–S bond functionalization of sulfones. Chem. Soc. Rev. 51, 6774–6823 (2022).
Li, S. & Shu, W. Recent advances in radical enabled selective Csp3–F bond activation of multifluorinated compounds. Chem. Commun. 58, 1066–1077 (2022).
Zhu, C. et al. Migratory functionalization of unactivated alkyl bromides for construction of all-carbon quaternary centers via transposed tert-C-radicals. Nat. Commun. 11, 4860 (2020).
Yue, W. J., Day, C. S. & Martin, R. Site-selective defluorinative sp3 C–H alkylation of secondary amides. J. Am. Chem. Soc. 143, 6395–6400 (2021).
Chen, F., Xu, X., He, Y., Huang, G. & Zhu, S. NiH-catalyzed migratory defluorinative olefin cross-coupling: trifluoromethyl-substituted alkenes as acceptor olefins to form gem-difluoroalkenes. Angew. Chem. Int. Ed. 59, 5398–5402 (2020).
Leriche, C., He, X., Chang, C.-W. T. & Liu, H.-W. Reversal of the apparent regiospecificity of NAD(P)H-dependent hydride transfer: the properties of the difluoromethylene group, a carbonyl mimic. J. Am. Chem. Soc. 125, 6348–6349 (2003).
Magueur, G., Crousse, B., Ourévitch, M., Bonnet-Delpon, D. & Bégué, J.-P. Fluoro-artemisinins: when a gem-difluoroethylene replaces a carbonyl group. J. Fluor. Chem. 127, 637–642 (2006).
Meanwell, N. A. Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 54, 2529–2591 (2011).
Li, Y. et al. Rhodium-catalyzed benzylic addition reactions of alkylarenes to Michael acceptors. Angew. Chem. Int. Ed. 61, e202207917 (2022).
Lo, J. C. et al. Fe-catalyzed C–C bond construction from olefins via radicals. J. Am. Chem. Soc. 139, 2484–2503 (2017).
Rakshit, A., Dhara, H. N., Sahoo, A. K. & Patel, B. K. The renaissance of organo nitriles in organic synthesis. Chem. Asian J. 17, e202200792 (2022).
Xu, Y., Zhang, M. & Oestreich, M. Photochemical, nickel-catalyzed C(sp3)–C(sp3) reductive cross-coupling of α-silylated alkyl electrophiles and allylic sulfones. ACS Catal. 12, 10546–10550 (2022).
Patra, T., Bellotti, P., Strieth-Kalthoff, F. & Glorius, F. Photosensitized intermolecular carboimination of alkenes through the persistent radical effect. Angew. Chem. Int. Ed. 59, 3172–3177 (2020).
Julia-Hernandez, F., Moragas, T., Cornella, J. & Martin, R. Remote carboxylation of halogenated aliphatic hydrocarbons with carbon dioxide. Nature 545, 84–88 (2017).
Zheng, S., Wang, W. & Yuan, W. Remote and proximal hydroaminoalkylation of alkenes enabled by photoredox/nickel dual catalysis. J. Am. Chem. Soc. 144, 17776–17782 (2022).
Sun, C., Li, Y. & Yin, G. Practical synthesis of chiral allylboronates by asymmetric 1,1-difunctionalization of terminal alkenes. Angew. Chem. Int. Ed. 61, e202209076 (2022).
Derosa, J., Apolinar, O., Kang, T., Tran, V. T. & Engle, K. M. Recent developments in nickel-catalyzed intermolecular dicarbofunctionalization of alkenes. Chem. Sci. 11, 4287–4296 (2020).
Wickham, L. M. & Giri, R. Transition metal (Ni, Cu, Pd)-catalyzed alkene dicarbofunctionalization reactions. Acc. Chem. Res. 54, 3415–3437 (2021).
Kang, T. et al. Nickel-catalyzed 1,2-carboamination of alkenyl alcohols. J. Am. Chem. Soc. 143, 13962–13970 (2021).
Ohno, K. et al. Evaluation of styrene oligomers eluted from polystyrene for estrogenicity in estrogen receptor binding assay, reporter gene assay, and uterotrophic assay. Food Chem. Toxicol. 41, 131–141 (2003).
Ellis, G. A., Wyche, T. P., Fry, C. G., Braun, D. R. & Bugni, T. S. Solwaric acids A and B, antibacterial aromatic acids from a marine Solwaraspora sp. Mar. Drugs 12, 1013–1022 (2014).
Fujita, T., Fuchibe, K. & Ichikawa, J. Transition-metal-mediated and -catalyzed C–F bond activation by fluorine elimination. Angew. Chem. Int. Ed. 58, 390–402 (2019).
Yao, C., Wang, S., Norton, J. & Hammond, M. Catalyzing the hydrodefluorination of CF3-substituted alkenes by PhSiH3. H· transfer from a nickel hydride. J. Am. Chem. Soc. 142, 4793–4799 (2020).
Lo, J. C., Gui, J., Yabe, Y., Pan, C. M. & Baran, P. S. Functionalized olefin cross-coupling to construct carbon–carbon bonds. Nature 516, 343–348 (2014).
Campbell, M. W., Yuan, M., Polites, V. C., Gutierrez, O. & Molander, G. A. Photochemical C–H activation enables nickel-catalyzed olefin dicarbofunctionalization. J. Am. Chem. Soc. 143, 3901–3910 (2021).
Zhang, C. S., Zhang, B. B., Zhong, L., Chen, X. Y. & Wang, Z. X. DFT insight into asymmetric alkyl–alkyl bond formation via nickel-catalysed enantioconvergent reductive coupling of racemic electrophiles with olefins. Chem. Sci. 13, 3728–3739 (2022).
Acknowledgements
The National Nature Science Foundation of China (22122107), the Fundamental Research Funds for Central Universities (2042022kf0026) and the Shanghai Committee of Science and Technology (grant number 21DZ1100100) are acknowledged for financial support. Y. Tong (Wuhan University) is kindly acknowledged for HRMS analysis. The numerical calculations in this paper were performed on the supercomputing system of the Super-computing Center of Wuhan University.
Author information
Authors and Affiliations
Contributions
G.Y. designed the project and directed the work. D.W., W.K., Y.B. and D.Z. performed all the synthetic experiments. Y.L. performed all the DFT calculations. G.Y. and D.W. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Mingbin Yuan and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods and References.
Supplementary Data 1
DFT coordinates for optimized structures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, D., Kong, W., Bao, Y. et al. Alkene 1,1-difunctionalizations via organometallic-radical relay. Nat Catal 6, 1030–1041 (2023). https://doi.org/10.1038/s41929-023-01032-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01032-0