Abstract
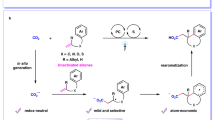
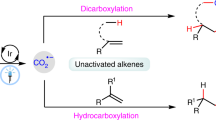
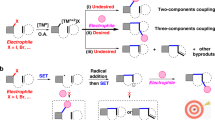
The conversion of CO2 to valuable chemicals is highly desirable for sustainable development. Among the various methods available, the difunctionalizing carboxylation of alkenes with CO2 emerges as an exceptionally efficient approach that facilitates the rapid construction of complex carboxylic acids and derivatives. However, existing systems have been limited to monocatalytic strategies and a few specific reaction types. Here we report a synergistic strategy to realize the aminocarboxylation of alkenes with CO2, providing efficient and practical synthetic access to valuable β-amino acids. The pivotal role of binaphthol as a photocatalyst in photoredox catalysis is demonstrated, enabling the single-electron activation of alkenes, generating radical anion intermediates. The remarkable aspect of this strategy lies in the efficient merger of photocatalysis and copper catalysis, enabling the rare orthogonal difunctionalization of alkene radical anions under redox-neutral conditions. Moreover, this strategy features mild conditions, high and unique selectivities, good functional group tolerance, facile product derivations and generality in realizing the hydroamination of alkenes.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Details about the materials and methods, experimental procedures, mechanistic studies, characterization data and NMR spectra are available in the Supplementary Information. Additional data are available from the corresponding author upon reasonable request.
References
Liu, Q., Wu, L., Jackstell, R. & Beller, M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 6, 5933 (2015).
Grignard, B., Gennen, S., Jérôme, C., Kleij, A. W. & Detrembleur, C. Advances in the use of CO2 as a renewable feedstock for the synthesis of polymers. Chem. Soc. Rev. 48, 4466–4514 (2019).
Tortajada, A., Juliá-Hernández, F., Börjesson, M., Moragas, T. & Martin, R. Transition-metal-catalyzed carboxylation reactions with carbon dioxide. Angew. Chem. Int. Ed. 57, 15948–15982 (2018).
Cao, Y., He, X., Wang, N., Li, H.-R. & He, L.-N. Photochemical and electrochemical carbon dioxide utilization with organic compounds. Chin. J. Chem. 36, 644–659 (2018).
Yeung, C. S. Photoredox catalysis as a strategy for CO2 incorporation: direct access to carboxylic acids from a renewable feedstock. Angew. Chem. Int. Ed. 58, 5492–5502 (2019).
Zhang, L., Li, Z., Takimoto, M. & Hou, Z. Carboxylation reactions with carbon dioxide using N-heterocyclic carbene-copper catalysts. Chem. Rec. 20, 494–512 (2020).
Cai, B., Cheo, H. W., Liu, T. & Wu, J. Light-promoted organic transformations utilizing carbon-based gas molecules as feedstocks. Angew. Chem. Int. Ed. 60, 18950–18980 (2021).
Ye, J.-H., Ju, T., Huang, H., Liao, L.-L. & Yu, D.-G. Radical carboxylative cyclizations and carboxylations with CO2. Acc. Chem. Res. 54, 2518–2531 (2021).
Liu, X.-F., Zhang, K., Tao, L., Lu, X.-B. & Zhang, W.-Z. Recent advances in electrochemical carboxylation reactions using carbon dioxide. Green. Chem. Eng. 3, 125–137 (2022).
Zhang, Z. et al. Radical-type difunctionalization of alkenes with CO2. Acta Chim. Sin. 77, 783–793 (2019).
Bertuzzi, G., Cerveri, A., Lombardi, L. & Bandini, M. Tandem functionalization–carboxylation reactions of π-systems with CO2. Chin. J. Chem. 39, 3116–3126 (2021).
Butcher, T. W. et al. Regioselective copper-catalyzed boracarboxylation of vinyl arenes. Org. Lett. 18, 6428–6431 (2016).
Chen, X. W. et al. Nickel-catalyzed asymmetric reductive carbo-carboxylation of alkenes with CO2. Angew. Chem. Int. Ed. 60, 14068–14075 (2021).
Takahashi, K., Sakurazawa, Y., Iwai, A. & Iwasawa, N. Catalytic synthesis of a methylmalonate salt from ethylene and carbon dioxide through photoinduced activation and photoredox-catalyzed reduction of nickelalactones. ACS Catal. 12, 3776–3781 (2022).
Yatham, V. R., Shen, Y. & Martin, R. Catalytic intermolecular dicarbofunctionalization of styrenes with CO2 and radical precursors. Angew. Chem. Int. Ed. 56, 10915–10919 (2017).
Ye, J.-H. et al. Visible-light-driven iron-promoted thiocarboxylation of styrenes and acrylates with CO2. Angew. Chem. Int. Ed. 56, 15416–15420 (2017).
Hou, J. et al. Visible-light-mediated metal-free difunctionalization of alkenes with CO2 and silanes or C(sp3)–H alkanes. Angew. Chem. Int. Ed. 57, 17220–17224 (2018).
Fu, Q. et al. Transition metal-free phosphonocarboxylation of alkenes with carbon dioxide via visible-light photoredox catalysis. Nat. Commun. 10, 3592 (2019).
Wang, H., Gao, Y., Zhou, C. & Li, G. Visible-light-driven reductive carboarylation of styrenes with CO2 and aryl halides. J. Am. Chem. Soc. 142, 8122–8129 (2020).
Ju, T. et al. Dicarboxylation of alkenes, allenes and (hetero)arenes with CO2 via visible-light photoredox catalysis. Nat. Catal. 4, 304–311 (2021).
Zhang, W. & Lin, S. Electroreductive carbofunctionalization of alkenes with alkyl bromides via a radical-polar crossover mechanism. J. Am. Chem. Soc. 142, 20661–20670 (2020).
Milligan, G., Shimpukade, B., Ulven, T. & Hudson, B. D. Complex pharmacology of free fatty acid receptors. Chem. Rev. 117, 67–110 (2017).
Pawar, V. et al. Rational design, synthesis and SAR study of novel warfarin analogous of 4-hydroxy coumarin-beta-aryl propanoic acid derivatives as potent anti-inflammatory agents. J. Mol. Struct. 1254, 132300 (2022).
Seo, H., Liu, A. & Jamison, T. F. Direct β-selective hydrocarboxylation of styrenes with CO2 enabled by continuous flow photoredox catalysis. J. Am. Chem. Soc. 139, 13969–13972 (2017).
Sheta, A. M. et al. Selective electrosynthetic hydrocarboxylation of α,β-unsaturated esters with carbon dioxide. Angew. Chem. Int. Ed. 60, 21832–21837 (2021).
Hendy, C. M., Smith, G. C., Xu, Z., Lian, T. & Jui, N. T. Radical chain reduction via carbon dioxide radical anion (CO2•−). J. Am. Chem. Soc. 143, 8987–8992 (2021).
Alektiar, S. N. & Wickens, Z. K. Photoinduced hydrocarboxylation via thiol-catalyzed delivery of formate across activated alkenes. J. Am. Chem. Soc. 143, 13022–13028 (2021).
Huang, Y. et al. Photoredox activation of formate salts: hydrocarboxylation of alkenes via carboxyl group transfer. ACS Catal. 11, 15004–15012 (2021).
Skubi, K. L., Blum, T. R. & Yoon, T. P. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 116, 10035–10074 (2016).
Twilton, J. et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 1, 0052 (2017).
Zhang, G., Cheng, Y., Beller, M. & Chen, F. Direct carboxylation with carbon dioxide via cooperative photoredox and transition‐metal dual catalysis. Adv. Synth. Catal. 363, 1583–1596 (2021).
Lu, F.-D., He, G.-F., Lu, L.-Q. & Xiao, W.-J. Metallaphotoredox catalysis for multicomponent coupling reactions. Green. Chem. 23, 5379–5393 (2021).
Xiong, T. & Zhang, Q. New amination strategies based on nitrogen-centered radical chemistry. Chem. Soc. Rev. 45, 3069–3087 (2016).
Zhao, Y. & Xia, W. Recent advances in radical-based C–N bond formation via photo-/electrochemistry. Chem. Soc. Rev. 47, 2591–2608 (2018).
Jiang, H. & Studer, A. Intermolecular radical carboamination of alkenes. Chem. Soc. Rev. 49, 1790–1811 (2020).
Cabrele, C., Martinek, T. A., Reiser, O. & Berlicki, L. Peptides containing β-amino acid patterns: challenges and successes in medicinal chemistry. J. Med. Chem. 57, 9718–9739 (2014).
Wang, S. & Xi, C. Recent advances in nucleophile-triggered CO2-incorporated cyclization leading to heterocycles. Chem. Soc. Rev. 48, 382–404 (2019).
Wang, L., Qi, C., Xiong, W. & Jiang, H. Recent advances in fixation of CO2 into organic carbamates through multicomponent reaction strategies. Chin. J. Catal. 43, 1598–1617 (2022).
Rulev, A. Y. Aza-Michael reaction: achievements and prospects. Russ. Chem. Rev. 80, 197–218 (2011).
Luan, Z. H., Qu, J. P. & Kang, Y. B. Discovery of oxygen α-nucleophilic addition to α,β-unsaturated amides catalyzed by redox-neutral organic photoreductant. J. Am. Chem. Soc. 142, 20942–20947 (2020).
Zhang, B. et al. Enantioselective diversification of alkene radical anions. Preprint at https://doi.org/10.26434/chemrxiv-2022-qkd7j (2022).
Senboku, H., Komatsu, H., Fujimura, Y. & Tokuda, M. Efficient electrochemical dicarboxylation of phenyl-substituted alkenes: synthesis of 1-phenylalkane-1,2-dicarboxylic acids. Synlett 3, 418–420 (2001).
Czyz, M. L., Taylor, M. S., Horngren, T. H. & Polyzos, A. Reductive activation and hydrofunctionalization of olefins by multiphoton tandem photoredox catalysis. ACS Catal. 11, 5472–5480 (2021).
Ran, C.-K. et al. Progress and challenges in dicarboxylation with CO2. Natl Sci. Open https://doi.org/10.1360/nso/20220024 (2023).
Venditto, N. J., Liang, Y. S., El Mokadem, R. K. & Nicewicz, D. A. Ketone–olefin coupling of aliphatic and aromatic carbonyls catalyzed by excited-state acridine radicals. J. Am. Chem. Soc. 144, 11888–11896 (2022).
Louvel, D. et al. Tailoring the reactivity of the Langlois reagent and styrenes with cyanoarenes organophotocatalysts under visible-light. Adv. Synth. Catal. 364, 139–148 (2022).
Li, Z.-L., Fang, G.-C., Gu, Q. S. & Liu, X. Y. Recent advances in copper-catalysed radical-involved asymmetric 1,2-difunctionalization of alkenes. Chem. Soc. Rev. 49, 32–48 (2020).
Zhang, Z., Chen, P. & Liu, G. Copper-catalyzed radical relay in C(sp3)–H functionalization. Chem. Soc. Rev. 51, 1640–1658 (2022).
Golden, D. L., Suh, S. E. & Stahl, S. S. Radical C(sp3)–H functionalization and cross-coupling reactions. Nat. Rev. Chem. 6, 405–427 (2022).
Liang, Y., Zhang, X. & MacMillan, D. W. C. Decarboxylative sp3 C–N coupling via dual copper and photoredox catalysis. Nature 559, 83–88 (2018).
Mao, R., Frey, A., Balon, J. & Hu, X. Decarboxylative C(sp3)–N cross-coupling via synergetic photoredox and copper catalysis. Nat. Catal. 1, 120–126 (2018).
Chen, C., Peters, J. C. & Fu, G. C. Photoinduced copper-catalysed asymmetric amidation via ligand cooperativity. Nature 596, 250–256 (2021).
Górski, B., Barthelemy, A.-L., Douglas, J. J., Juliá, F. & Leonori, D. Copper-catalysed amination of alkyl iodides enabled by halogen-atom transfer. Nat. Catal. 4, 623–630 (2021).
Bartolomei, B., Gentile, G., Rosso, C., Filippini, G. & Prato, M. Turning the light on phenols: new opportunities in organic synthesis. Chem. Eur. J. 27, 16062–16070 (2021).
Schmalzbauer, M. et al. Redox-neutral photocatalytic C–H carboxylation of arenes and styrenes with CO2. Chem 6, 2658–2672 (2020).
Liu, C., Shen, N. & Shang, R. Photocatalytic defluoroalkylation and hydrodefluorination of trifluoromethyls using o-phosphinophenolate. Nat. Commun. 13, 354 (2022).
Liang, K. et al. Deprotection of benzyl-derived groups via photochemically mesolytic cleavage of C–N and C–O bonds. Chem 9, 511–522 (2023).
Lu, M.-Z. et al. Recent advances in alkenyl sp2 C–H and C–F bond functionalizations: scope, mechanism, and applications. Chem. Rev. 122, 17479–17646 (2022).
Lu, W. & Shen, Z. Direct synthesis of alkenylboronates from alkenes and pinacol diboron via copper catalysis. Org. Lett. 21, 142–146 (2019).
Hossain, A., Bhattacharyya, A. & Reiser, O. Copper’s rapid ascent in visible-light photoredox catalysis. Science 364, eaav9713 (2019).
Zheng, Y.-W., Narobe, R., Donabauer, K., Yakubov, S. & König, B. Copper(II)-photocatalyzed N–H alkylation with alkanes. ACS Catal. 10, 8582–8589 (2020).
Cho, H., Suematsu, H., Oyala, P. H., Peters, J. C. & Fu, G. C. Photoinduced, copper-catalyzed enantioconvergent alkylations of anilines by racemic tertiary electrophiles: synthesis and mechanism. J. Am. Chem. Soc. 144, 4550–4558 (2022).
Lu, D. et al. Cu-catalyzed C(sp3)–N coupling and alkene carboamination enabled by ligand-promoted selective hydrazine transfer to alkyl radicals. ACS Catal. 12, 3269–3278 (2022).
Acknowledgements
Financial support is provided by the National Natural Science Foundation of China (22225106; to D.-G.Y.), Fundamental Research Funds from Sichuan University (2020SCUNL102; to D.-G.Y.) and the Fundamental Research Funds for the Central Universities. We also thank X. Wang from the Analysis and Testing Center of Sichuan University as well as J. Li, Q. Zhang and D. Deng from the College of Chemistry at Sichuan University for compound testing.
Author information
Authors and Affiliations
Contributions
D.-G.Y., J.-H.Y. and J.-P.Y. conceived and designed the study. J.-P.Y., J.-H.Y., D.-G.Y. and L.Y. wrote the paper. J.-P.Y., J.-C.X., H.-T.L., X.-W.C., H.-X.S. and Y.D. performed the experiments and mechanistic studies. All authors contributed to the analysis and interpretation of the data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare the following competing financial interest(s): a Chinese patent on this work has been applied with the number 202211731369.0 (D.-G.Y., J.-P.Y., J.-C.X., H.-T.L., H.-X.S., Y.D., X.-W.C. and J.-H.Y.).
Peer review
Peer review information
Nature Catalysis thanks Jie Wu for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Tables 1 and 2, Figs. 1–16 and refs. 1–22.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yue, JP., Xu, JC., Luo, HT. et al. Metallaphotoredox-enabled aminocarboxylation of alkenes with CO2. Nat Catal 6, 959–968 (2023). https://doi.org/10.1038/s41929-023-01029-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01029-9
This article is cited by
-
Modular synthesis of β-amino acids from alkenes, amines, and CO2 via photoredox/copper dual catalysis
Science China Chemistry (2023)