Abstract
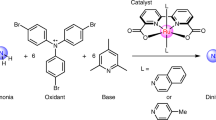
A century after hydrazine was discovered, its synthesis remains a challenge due to its high energy requirement and favourable subsequent conversion. As an alternative to the traditional industrial processes, the direct electrochemical oxidation of ammonia is an ideal reaction for hydrazine synthesis. However, suitable methods still need to be developed. Here we show that ruthenium complexes bearing 2-[5-(pyridin-2-yl)-1H-pyrrol-2-yl]pyridine ligands display high catalytic activity for the direct electrochemical oxidation of ammonia to generate hydrazine in acetonitrile. Our developed CSU-2 complex reached a turnover number for hydrazine formation of 5,735 within 24 h at an applied potential of 1.0 V versus Cp2Fe+/0. The results reveal a bimolecular N‒N coupling mechanism involving RuII–aminyl or RuIII–iminyl intermediates.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2158154 (CSU-1) and 2158155 (CSU-2). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper. All other data supporting the findings of this study are available within the article and its supplementary information or from the corresponding author upon request.
References
Schirmann, J. P. & Bourdauducq, P. in Ullmann’s Encyclopedia of Industrial Chemistry Vol. 18, 79–96 (Wiley, 2012).
Rothgery, E. F. in Kirk-Othmer Encyclopedia of Chemical Technology Vol. 13, 562–607 (Wiley, 2004).
Elishav, O. et al. Progress and prospective of nitrogen-based alternative fuels. Chem. Rev. 120, 5352–5436 (2020).
Serov, A. et al. Anode catalyst for direct hydrazine fuel cells: from laboratory test to an electric vehicle. Angew. Chem. Int. Ed. 53, 10336–10339 (2014).
Raschig, F. Vorlesungsversuche aus der Chemie der anorganischen Stickstoffverbindungen. Ber. Dtsch. Chem. Ges. 40, 4587 (1907).
Wang, F., Gerken, J. B., Bates, D. M., Kim, Y. J. & Stahl, S. S. Electrochemical strategy for hydrazine synthesis: development and overpotential analysis of methods for oxidative N–N coupling of an ammonia surrogate. J. Am. Chem. Soc. 142, 12349–12356 (2020).
Cheddie, D. Hydrogen Energy—Challenges And Perspectives (ed. Minić, D.) Ch. 13 (IntechOpen, 2012).
Afif, A. et al. Ammonia-fed fuel cells: a comprehensive review. Renew. Sust. Energ. Rev. 60, 822–835 (2016).
Adli, N. M., Zhang, H., Mukherjee, S. & Wu, G. Review—ammonia oxidation electrocatalysis for hydrogen generation and fuel cells. J. Electrochem. Soc. 165, J3130–J3147 (2018).
Nakajima, K., Toda, H., Sakata, K. & Nishibayashi, Y. Ruthenium-catalysed oxidative conversion of ammonia into dinitrogen. Nat. Chem. 11, 702–709 (2019).
Bhattacharya, P. et al. Catalytic ammonia oxidation to dinitrogen by hydrogen atom abstraction. Angew. Chem. Int. Ed. 58, 11618–11624 (2019).
Habibzadeha, F., Millera, S. L., Hamanna, T. W. & Smith, M. R. III Homogeneous electrocatalytic oxidation of ammonia to N2 under mild conditions. Proc. Nat. Acad. Sci. USA 116, 2849–2853 (2019).
Dunn, P. L., Johnson, S. I., Kaminsky, W. & Bullock, R. M. Diversion of catalytic C–N bond formation to catalytic oxidation of NH3 through modification of the hydrogen atom absorption. J. Am. Chem. Soc. 142, 3361–3365 (2020).
Holub, J. et al. Synthesis, structure and ammonia oxidation catalytic activity of Ru-NH3 complexes containing multidentate polypyridyl ligands. Inorg. Chem. 60, 13929–13940 (2021).
Trenerry, M. J., Wallen, C. M., Brwon, T. R., Park, S. V. & Berry, J. F. Spontaneous N2 formation by a diruthenium complex enables electrocatalytic and aerobic oxidation of ammonia. Nat. Chem. 13, 1221–1227 (2021).
Zott, M. D., Garrido-Barros, P. & Peters, J. C. Electrocatalytic ammonia oxidation mediated by a polypyridyl iron catalyst. ACS Catal. 9, 10101–10108 (2019).
Zott, M. D. & Peters, J. C. Enhanced ammonia oxidation catalysis by a low-spin iron complex featuring cis-coordination sites. J. Am. Chem. Soc. 143, 7612–7616 (2021).
Li, Y. et al. A parent iron amido complex in catalysis in catalysis of ammonia oxidation. J. Am. Chem. Soc. 144, 4365–4375 (2022).
Toda, H. et al. Manganese-catalyzed ammonia oxidation into dinitrogen under chemical or electrochemical conditions. ChemPlusChem 86, 1511–1516 (2021).
Liu, H.-Y. et al. Electrocatalytic, homogeneous ammonia oxidation in water to nitrate and nitrite with a copper complex. J. Am. Chem. Soc. 144, 8449–8453 (2022).
Najafian, A. & Cundari, T. R. Computational mechanistic study of electro-oxidation of ammonia to N2 by homogenous ruthenium and iron complexes. J. Phys. Chem. A. 123, 7973–7982 (2019).
Dunn, P. L., Cook, B. J., Jobnson, S. I., Appel, A. M. & Bullock, R. M. Oxidation of ammonia with molecular complexes. J. Am. Chem. Soc. 142, 17845–17858 (2020).
Hayashi, H. Hydrazine synthesis by a catalytic oxidation process. Catal. Rev. Sci. Eng. 32, 229–277 (1990).
Hayashi, H., Kainoh, A., Katayama, M., Kawasaki, K. & Okazaki, T. Hydrazine production from ammonia via azine. Ind. Eng. Chem. Prod. Res. Dev. 15, 299–303 (1976).
Gardner, E. J. et al. Uncovering redox non-innocent hydrogen-bonding in Cu(I)-diazene complexes. J. Am. Chem. Soc. 143, 15960–15974 (2021).
McSkimming, A. et al. An easy one-pot synthesis of diverse 2,5-di(2-pyridyl)pyrroles: a versatile entry point to metal complexes of functionalized, meridial and tridentate 2,5-di(2-pyridyl)pyrrolato ligands. Chem. Eur. J. 20, 11445–11456 (2014).
Trenerry, M. J., Wallen, C. M., Brown, T. R., Park, S. V. & Berry, J. F. Spontaneous N2 formation by a diruthenium complex enables electrocatalytic and aerobic oxidation of ammonia. Nat. Chem. 13, 1221–1227 (2021).
Watt, G. W. & Chrisp, J. D. Spectrophotometric method for determination of hydrazine. Anal. Chem. 24, 2006–2008 (1952).
Gu, N. X., Oyala, F. H. & Peters, J. C. Hydrazine formation via coupling of a nickel(III)–NH2 radical. Angew. Chem. Int. Ed. 60, 4009–4013 (2021).
Kundu, S., Dutta, D., Maity, S., Weyhermüller, T. & Ghosh, P. Proton-coupled oxidation of a diarylamine: amido and aminyl radical complexes of ruthenium(II). Inorg. Chem. 57, 11978–11960 (2018).
Olivos Suarez, A. I., Lyaskovskyy, V., Reek, J. N. H., Vlugt, J. I. & Bruin, B. Complexes with nitrogen-centered radical ligands: classification, spectroscopic features, reactivity, and catalytic applications. Angew. Chem. Int. Ed. 52, 12510–12529 (2013).
Collman, J. P., Hutchison, J. E., Ennis, M. S., Lopez, M. A. & Guilard, R. Reduced nitrogen hydride complexes of a cofacial metallodiporphyrin and their oxidative interconversion. An analysis of ammonia oxidation and prospects for a dinitrogen electroreduction catalyst based on cofacial metallodiporphyrins. J. Am. Chem. Soc. 114, 8074–8080 (1992).
Sheldrick, G. M. SADABS. Program for empirical absorption correction of area detector data (Univ. Göttingen, 1996).
SHELXL Manual (Bruker AXS, 1997).
Chen, G. et al. Efficient electrochemical water oxidation mediated by pyridylpyrrole-carboxylate ruthenium complexes. Inorg. Chem. 60, 15627–15634 (2021).
Chen, G. et al. A Ru2II,III diphosphonato complex with a metal–metal bond for water oxidation. Dalton Trans. 50, 2018–2022 (2021).
Yang, J. et al. From Ru-bda to Ru-bds: a step forward to highly efficient molecular water oxidation electrocatalysts under acidic and neutral conditions. Nat. Commun. 12, 373 (2021).
Shen, J., Wang, M., Zhang, P., Jiang, J. & Sun, L. Electrocatalytic water oxidation by copper(ii) complexes containing a tetra- or pentadentate amine-pyridine ligand. Chem. Commun. 53, 4374–4377 (2017).
Chen, Q. et al. CoO nanoparticle decorated N-doped carbon nanotubes: a high-efficiency catalyst for nitrate reduction to ammonia. Chem. Commun. 58, 5091–5904 (2022).
Zhu, D., Zhang, L., Ruther, R. E. & Hamers, R. J. Photo-illuminated diamond as a solid-state source of solvated electrons in water for nitrogen reduction. Nat. Mater. 12, 836–841 (2013).
Frisch, M. J. et al. Gaussian 16, Revision C.01 (Gaussian Inc., 2016).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0. model. J. Chem. Phys. 110, 6158–6170 (1999).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 8, 1057–1065 (2006).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Acknowledgements
We thank G.-L. Liang from Southeast University, Z.-M. Yang from Nankai University and Q.-G. Wang from Tongji University for their encouragement, motivation and very fruitful discussions. We also thank Y.-W. Lin from the University of South China, L. Yu from the High Magnetic Field Laboratory of the Chinese Academy of Sciences for the EPR measurement and discussion and Z.-C. Zhang from Huaiyin Normal University for refinement of single crystal structures. X.-Y.Y. acknowledges the National Natural Science Foundation of China (21571190), the Foundation of Central South University Research Programme of Advanced Interdisciplinary (2023QYJC019) and the Natural Science Foundation of Hunan Province of China (2022JJ30689). G.C. thanks the Postgraduate Scientific Research Innovation Project of Hunan Province (CX20210170).
Author information
Authors and Affiliations
Contributions
X.-Y.Y. as the corresponding author contributed to project design and paper revision. G.C. mainly contributed to synthesis and electrocatalysis studies of the CSU complexes and writing the paper. P.H. mainly contributed to the DFT calculations. C.L. mainly contributed to X-ray diffraction analysis of the solid-state structures. X.-F.M., J.-J.W., Z.-W.C., T.C. and L.-Z.F. assisted with synthesis and characterization of complexes, CPC experiments and gas chromatography experiments and so on.
Corresponding author
Ethics declarations
Competing interests
Y.-X.Y. and G.C. have filed patents based on the work described here (China patent application no. 202111584527.X, international application no. PCT/CN2022/139184, US patent application no. 18272369, European patent application no. 22909854.6, Korean patent application no. 10-2023-7024287, Japanese patent application no. 2023-524432).
Peer review
Peer review information
Nature Catalysis thanks Jeffrey W. Bacon, Victor Batista, Ignacio Funes-Ardoiz, Sungho Park and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–82, Tables 1–4 and Note 1.
Supplementary Video 1
H2, N2H4 and N2 production in CPC experiments. Electrolytic conditions: 0.01 mM CSU-2 in NH3-saturated MeCN solutions at a constant potential of 0.76 V versus Cp2Fe+/0 at room temperature.
Supplementary Data 1
The atomic coordinates of the optimized models of CSU-1, CSU-2 and intermediates.
Supplementary Data 2
cif file of CSU-1.
Supplementary Data 3
cif file of CSU-2.
Source data
Source Data Fig. 2
Source data of Fig. 2
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, G., He, P., Liu, C. et al. Direct synthesis of hydrazine by efficient electrochemical ruthenium-catalysed ammonia oxidation. Nat Catal 6, 949–958 (2023). https://doi.org/10.1038/s41929-023-01025-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01025-z
This article is cited by
-
Defying thermodynamics to synthetize hydrazine
Nature Catalysis (2023)