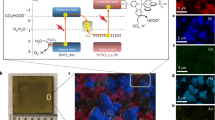
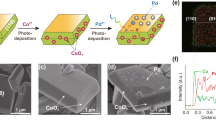
Abstract
Generation of C2+ compounds from sunlight, carbon dioxide and water provides a promising path for carbon neutrality. Central to the construction of a rational artificial photosynthesis integrated device is the requirement for a catalyst to break the bottleneck of C–C coupling. Here, based on operando spectroscopy measurements, theoretical calculations and feedstock experiments, it is discovered that gold, in conjunction with iridium, can catalyse the reduction of CO2, achieving C–C coupling by insertion of CO2 into –CH3. Due to a combination of optoelectronic and catalytic properties, the assembly of AuIr with InGaN nanowires on silicon enables the achievement of a C2H6 activity of 58.8 mmol g−1 h−1 with a turnover number of 54,595 over 60 h. A light-to-fuel efficiency of ~0.59% for solar fuel production from CO2 and H2O is achieved without any other energy inputs. This work provides a carbon-negative path for producing higher-order carbon compounds.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Supporting data are available at the University of Michigan (https://doi.org/10.7302/w8ep-0g79). Further details regarding the data are available from the authors upon reasonable request.
References
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Zhang, B. & Sun, L. Artificial photosynthesis: opportunities and challenges of molecular catalysts. Chem. Soc. Rev. 48, 2216–2264 (2019).
Pinaud, B. A. et al. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy Environ. Sci. 6, 1983–2002 (2013).
Lee, B.-H. et al. Electronic interaction between transition metal single-atoms and anatase TiO2 boosts CO2 photoreduction with H2O. Energy Environ. Sci. 15, 601–609 (2022).
Ali, S., Razzaq, A., Kim, H. & In, S.-I. Activity, selectivity, and stability of Earth-abundant CuO/Cu2O/Cu0-based photocatalysts toward CO2 reduction. Chem. Eng. J. 429, 131579 (2022).
Wang, L. et al. Surface strategies for catalytic CO2 reduction: from two-dimensional materials to nanoclusters to single atoms. Chem. Soc. Rev. 48, 5310–5349 (2019).
Habisreutinger, S. N., Schmidt-Mende, L. & Stolarczyk, J. K. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Ed. 52, 7372–7408 (2013).
Liu, W. et al. A scalable general synthetic approach toward ultrathin imine-linked two-dimensional covalent organic framework nanosheets for photocatalytic CO2 reduction. J. Am. Chem. Soc. 141, 17431–17440 (2019).
Wang, S. L. et al. Porous hypercrosslinked polymer–TiO2–graphene composite photocatalysts for visible-light-driven CO2 conversion. Nat. Commun. 10, 10 (2019).
Reisner, E. et al. Molecularly engineered photocatalyst sheet for scalable solar formate production from carbon dioxide and water. Nat. energy 5, 703–710 (2020).
Wang, Y. et al. Visible-light driven overall conversion of CO2 and H2O to CH4 and O2 on 3D-SiC@2D-MoS2 heterostructure. J. Am. Chem. Soc. 140, 14595–14598 (2018).
Iwase, A. et al. Water splitting and CO2 reduction under visible light irradiation using Z-scheme systems consisting of metal sulfides, CoOx-loaded BiVO4, and a reduced graphene oxide electron mediator. J. Am. Chem. Soc. 138, 10260–10264 (2016).
Andrei, V., Reuillard, B. & Reisner, E. Bias-free solar syngas production by integrating a molecular cobalt catalyst with perovskite-BiVO4 tandems. Nat. Mater. 19, 189–194 (2020).
Zhou, B. et al. Mo–Bi–Cd ternary metal chalcogenides: highly efficient photocatalyst for CO2 reduction to formic acid under visible light. ACS Sustain. Chem. Eng. 6, 5754–5759 (2018).
Kibria, M. G. et al. Atomic-scale origin of long-term stability and high performance of p-GaN nanowire arrays for photocatalytic overall pure water splitting. Adv. Mater. 28, 8388–8397 (2016).
Li, X. et al. Selective visible-light-driven photocatalytic CO2 reduction to CH4 mediated by atomically thin CuIn5S8 layers. Nat. Energy 4, 690–699 (2019).
Hao, L. et al. Surface-halogenation-induced atomic-site activation and local charge separation for superb CO2 photoreduction. Adv. Mater. 31, 1900546 (2019).
Cestellos-Blanco, S., Zhang, H., Kim, J. M., Shen, Y.-X. & Yang, P. Photosynthetic semiconductor biohybrids for solar-driven biocatalysis. Nat. Catal. 3, 245–255 (2020).
Lin, S. et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 349, 1208–1213 (2015).
Chu, S. et al. Tunable syngas production from CO2 and H2O in an aqueous photoelectrochemical cell. Angew. Chem. Int. Ed. 55, 14260–14264 (2016).
Chu, S. et al. Photoelectrochemical CO2 reduction into syngas with the metal/oxide interface. J. Am. Chem. Soc. 140, 7869–7877 (2018).
Hori, Y., Takahashi, I., Koga, O. & Hoshi, N. Selective formation of C2 compounds from electrochemical reduction of CO2 at a series of copper single crystal electrodes. J. Phys. Chem. B 106, 15–17 (2002).
Wang, Y. et al. Catalyst synthesis under CO2 electroreduction favours faceting and promotes renewable fuels electrosynthesis. Nat. Catal. 3, 98–106 (2020).
Jeon, H. S., Kunze, S., Scholten, F. & Roldan Cuenya, B. Prism-shaped Cu nanocatalysts for electrochemical CO2 reduction to ethylene. ACS Catal. 8, 531–535 (2018).
Jung, H. et al. Electrochemical fragmentation of Cu2O nanoparticles enhancing selective C–C coupling from CO2 reduction reaction. J. Am. Chem. Soc. 141, 4624–4633 (2019).
Kibria, M. et al. Visible light-driven efficient overall water splitting using p-type metal-nitride nanowire arrays. Nat. Commun. 6, 1–8 (2015).
Li, Y. X., Sadaf, S. M. & Zhou, B. W. Ga(X)N/Si nanoarchitecture: an emerging semiconductor platform for sunlight-powered water splitting toward hydrogen. Front. Energy https://doi.org/10.1007/s11708-023-0881-9 (2023).
AlOtaibi, B., Fan, S. Z., Wang, D. F., Ye, J. H. & Mi, Z. T. Wafer-level artificial photosynthesis for CO2 reduction into CH4 and CO using GaN nanowires. ACS Catal. 5, 5342–5348 (2015).
Kauffman, D. R., Alfonso, D., Matranga, C., Qian, H. & Jin, R. Experimental and computational investigation of Au25 clusters and CO2: a unique interaction and enhanced electrocatalytic activity. J. Am. Chem. Soc. 134, 10237–10243 (2012).
Back, S., Yeom, M. S. & Jung, Y. Active sites of Au and Ag nanoparticle catalysts for CO2 electroreduction to CO. ACS Catal. 5, 5089–5096 (2015).
Guan, X. et al. Making of an industry-friendly artificial photosynthesis device. ACS Energy Lett. 3, 2230–2231 (2018).
Tran, P. D., Wong, L. H., Barber, J. & Loo, J. S. C. Recent advances in hybrid photocatalysts for solar fuel production. Energy Environ. Sci. 5, 5902–5918 (2012).
Sreedhar, A. et al. Charge carrier generation and control on plasmonic Au clusters functionalized TiO2 thin films for enhanced visible light water splitting activity. Ceram. Int. 44, 18978–18986 (2018).
Wang, Y. X. et al. Deep ultraviolet light source from ultrathin GaN/AlN MQW structures with output power over 2 watt. Adv. Opt. Mater. 7, 7 (2019).
Hafiz, S. et al. Determination of carrier diffusion length in GaN. J. Appl. Phys. 117, 013106 (2015).
Zhou, B. et al. Gallium nitride nanowire as a linker of molybdenum sulfides and silicon for photoelectrocatalytic water splitting. Nat. Commun. 9, 3856 (2018).
Zhou, B. et al. Highly efficient binary copper–iron catalyst for photoelectrochemical carbon dioxide reduction toward methane. Proc. Natl Acad. Sci. USA 117, 1330–1338 (2020).
Chen, Y., Li, C. W. & Kanan, M. W. Aqueous CO2 reduction at very low overpotential on oxide-derived Au nanoparticles. J. Am. Chem. Soc. 134, 19969–19972 (2012).
Liu, M. et al. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature 537, 382–386 (2016).
Mistry, H. et al. Exceptional size-dependent activity enhancement in the electroreduction of CO2 over Au nanoparticles. J. Am. Chem. Soc. 136, 16473–16476 (2014).
Zhang, P., Wang, S., Guan, B. Y. & Lou, X. W. Fabrication of CdS hierarchical multi-cavity hollow particles for efficient visible light CO2 reduction. Energy Environ. Sci. 12, 164–168 (2019).
Vanka, S. et al. Long-term stability studies of a semiconductor photoelectrode in three-electrode configuration. J. Mater. Chem. A 7, 27612–27619 (2019).
Kibria, M. G. et al. Atomic-scale origin of long-term stability and high performance of p-GaN nanowire arrays for photocatalytic overall water splitting. Adv. Mater. 2016, 8388–8397 (2016).
Montoya, J. H., Shi, C., Chan, K. & Nørskov, J. K. Theoretical insights into a CO dimerization mechanism in CO2 electroreduction. J. Phys. Chem. Lett. 6, 2032–2037 (2015).
Zhou, Y. S. et al. Dopant-induced electron localization drives CO2 reduction to C2 hydrocarbons. Nat. Chem. 10, 974–980 (2018).
Zhao, Y. et al. Direct C–C coupling of CO2 and the methyl group from CH4 activation through facile insertion of CO2 into Zn–CH3 σ-bond. J. Am. Chem. Soc. 138, 10191–10198 (2016).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Hafner, J. Abinitio molecular-dynamics for liquid-metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Hafner, J. Ab-initio molecular-dynamics simulation of the liquid-metal amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Grimme, S. et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Garcia, S. et al. Microwave synthesis of classically immiscible rhodium–silver and rhodium–gold alloy nanoparticles: highly active hydrogenation catalysts. ACS Nano 8, 11512–11521 (2014).
Luo, L. et al. Tunability of the adsorbate binding on bimetallic alloy nanoparticles for the optimization of catalytic hydrogenation. J. Am. Chem. Soc. 139, 5538–5546 (2017).
Acknowledgements
The work performed at the University of Michigan has been supported by the College of Engineering Blue Sky Research Initiative at the University of Michigan and United States Army Research Office Award W911NF2110337. B.Z. is grateful for financial support by the College of Engineering Blue Sky Research Initiative during his postdoctoral studies at the University of Michigan. J.S. and P.O. acknowledge financial support by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (grant number NSERC RGPIN-2017-05187) and thank Compute Canada for providing computing resources. J.K.C. acknowledges support by the Liquid Sunlight Alliance (transient reflection spectroscopy), which is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, Fuels from Sunlight Hub under award number DE-SC0021266.
Author information
Authors and Affiliations
Contributions
B.Z. and Z.M. conceived this project. B.Z., Z.Y., Y.M., P.W. and P.Z. conducted the co-catalyst deposition, APID characterizations, photocatalytic CO2 reduction experiments and data analysis. P.O. conducted the DFT calculations with the assistance of X.-Y.L. and J.S. B.Z and Z.M. participated in discussion of the theoretical calculations. S.V. conducted the epitaxial growth of InGaN NWs with the assistance of Y.X. and I.A.N. T.M. performed the STEM characterization. H.S. collected the DRIFT spectra. J.K.C. carried out the transient reflection spectroscopy measurements and analysed these data. J.P. participated in analysis and discussion. B.Z., Z.Y., Y.M., P.O., J.S. and Z.M. wrote the paper with contributions from all the authors.
Corresponding authors
Ethics declarations
Competing interests
A US provisional patent has been filed based on this work by the inventors of Z.M., Z.Y., Y.M. and B.Z. Some intellectual property related to this work is licensed to NS Nanotech, Inc. and NX Fuels, Inc., which were co-founded by Z.M. The University of Michigan and Z.M. have a financial interest in these companies.
Peer review
Peer review information
Nature Catalysis thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–28 and Tables 1 and 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, B., Ma, Y., Ou, P. et al. Light-driven synthesis of C2H6 from CO2 and H2O on a bimetallic AuIr composite supported on InGaN nanowires. Nat Catal 6, 987–995 (2023). https://doi.org/10.1038/s41929-023-01023-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01023-1
This article is cited by
-
Modulation in the electronic structure of Ir-rich shell on AuIr solid solution as OER electrocatalyst for PEM electrolyzer
Journal of Applied Electrochemistry (2024)
-
A Unified View of Carbon Neutrality: Solar-Driven Selective Upcycling of Waste Plastics
Transactions of Tianjin University (2024)
-
Photocatalytic reduction of CO2 with H2O into C2H6 mediated by dual metalation strategy
Science China Chemistry (2023)