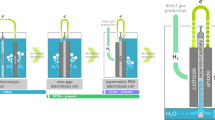
Abstract
Acidic electrochemical CO2 reduction (CO2R) addresses CO2 loss and thus mitigates the energy penalties associated with CO2 recovery; however, acidic CO2R suffers low selectivity. One promising remedy—using a high concentration of alkali cations—steers CO2R towards multi-carbon (C2+) products, but these same alkali cations result in salt formation, limiting operating stability to <15 h. Here we present a copper catalyst functionalized with cationic groups (CG) that enables efficient CO2 activation in a stable manner. By replacing alkali cations with immobilized benzimidazolium CG within ionomer coatings, we achieve over 150 h of stable CO2R in acid. We find the water-management property of CG minimizes proton migration that enables operation at a modest voltage of 3.3 V with mildly alkaline local pH, leading to more energy-efficient CO2R with a C2+ Faradaic efficiency of 80 ± 3%. As a result, we report an energy efficiency of 28% for acidic CO2R towards C2+ products and a single-pass CO2 conversion efficiency exceeding 70%.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Source data for the stability test shown in Fig. 4a,b and the atomic coordinates of the optimized computational models can be found in figshare26. Data that support the findings of this study can be found in the article and Supplementary information. All other data supporting this work are available from the corresponding authors upon reasonable request.
References
Li, L., Li, X., Sun, Y. & Xie, Y. Rational design of electrocatalytic carbon dioxide reduction for a zero-carbon network. Chem. Soc. Rev. 51, 1234–1252 (2022).
Kibria Nabil, S., McCoy, S. & Kibria, M. G. Comparative life cycle assessment of electrochemical upgrading of CO2 to fuels and feedstocks. Green Chem. 23, 867–880 (2021).
Artz, J. et al. Sustainable conversion of carbon dioxide: an integrated review of catalysis and life cycle assessment. Chem. Rev. 118, 434–504 (2018).
Pelayo García de Arquer, F. et al. CO2 electrolysis to multi-carbon products at activities greater than 1 A cm−2. Science 367, 661–666 (2020).
Gabardo, C. M. et al. Continuous carbon dioxide electroreduction to concentrated multi-carbon products using a membrane electrode assembly. Joule 3, 2777–2791 (2019).
Wang, Y. et al. Catalyst synthesis under CO2 electroreduction favours faceting and promotes renewable fuels electrosynthesis. Nat. Catal. 3, 98–106 (2019).
Xu, H. et al. Highly selective electrocatalytic CO2 reduction to ethanol by metallic clusters dynamically formed from atomically dispersed copper. Nat. Energy 5, 623–632 (2020).
Ma, M. et al. Insights into the carbon balance for CO2 electroreduction on Cu using gas diffusion electrode reactor designs. Energy Environ. Sci. 13, 977–985 (2020).
Larrazabal, G. O. et al. Analysis of mass flows and membrane cross-over in CO2 reduction at high current densities in an MEA-type electrolyzer. ACS Appl. Mater. Interfaces 11, 41281–41288 (2019).
Ozden, A. et al. Cascade CO2 electroreduction enables efficient carbonate-free production of ethylene. Joule 5, 706–719 (2021).
Blommaert, M. A., Subramanian, S., Yang, K., Smith, W. A. & Vermaas, D. A. High indirect energy consumption in AEM-based CO2 electrolyzers demonstrates the potential of bipolar membranes. ACS Appl. Mater. Interfaces 14, 557–563 (2022).
Huang, J. E. et al. CO2 electrolysis to multi-carbon products in strong acid. Science 372, 1074–1078 (2021).
Gu, J. et al. Modulating electric field distribution by alkali cations for CO2 electroreduction in strongly acidic medium. Nat. Catal. 5, 268–276 (2022).
Xie, Y. et al. High carbon utilization in CO2 reduction to multi-carbon products in acidic media. Nat. Catal. 5, 564–570 (2022).
Monteiro, M. C. O. et al. Absence of CO2 electroreduction on copper, gold and silver electrodes without metal cations in solution. Nat. Catal. 4, 654–662 (2021).
Monteiro, M. C. O., Dattila, F., Lopez, N. & Koper, M. T. M. The role of cation acidity on the competition between hydrogen evolution and CO2 reduction on gold electrodes. J. Am. Chem. Soc. 144, 1589–1602 (2021).
Xu, Y. et al. Self-cleaning CO2 reduction systems: unsteady electrochemical forcing enables stability. ACS Energy Lett. 6, 809–815 (2021).
Endrődi, B. et al. Operando cathode activation with alkali metal cations for high current density operation of water-fed zero-gap carbon dioxide electrolysers. Nat. Energy 6, 439–448 (2021).
Ren, W., Xu, A., Chan, K. & Hu, X. A cation concentration gradient approach to tune the selectivity and activity of CO2 electroreduction. Angew. Chem. Int. Ed. 61, e2022141 (2022).
Ma, Z. et al. CO2 electroreduction to multi-carbon products in strongly acidic electrolyte via synergistically modulating the local microenvironment. Nat. Commun. 13, 7596 (2022).
Resasco, J. et al. Promoter effects of alkali metal cations on the electrochemical reduction of carbon dioxide. J. Am. Chem. Soc. 139, 11277–11287 (2017).
Ummireddi, A. K., Sharma, S. K. & Pala, R. G. S. Ammonium ionic liquid cation promotes electrochemical CO2 reduction to ethylene over formate while inhibiting the hydrogen evolution on a copper electrode. Catal. Sci. Technol. 12, 519–529 (2022).
Pankhurst, J. R., Guntern, Y. T., Mensi, M. & Buonsanti, R. Molecular tunability of surface-functionalized metal nanocrystals for selective electrochemical CO2 reduction. Chem. Sci. 10, 10356–10365 (2019).
Zhong, S. et al. Efficient electrochemical transformation of CO2 to C2/C3 chemicals on benzimidazole-functionalized copper surfaces. Chem. Commun. 54, 11324–11327 (2018).
Banerjee, S., Gerke, C. S. & Thoi, V. S. Guiding CO2RR selectivity by compositional tuning in the electrochemical double layer. Acc. Chem. Res. 55, 504–515 (2022).
Fan, M. et al. Cationic-group functionalized electrocatalysts enable stable acidic CO2 electrolysis. figshare https://doi.org/10.6084/m9.figshare.22819415 (2023).
Wright, A. G. et al. Hexamethyl-p-terphenyl poly(benzimidazolium): a universal hydroxide-conducting polymer for energy conversion devices. Energy Environ. Sci. 9, 2130–2142 (2016).
Fan, J. et al. Poly(bis-arylimidazoliums) possessing high hydroxide ion exchange capacity and high alkaline stability. Nat. Commun. 10, 2306 (2019).
Fan, J. et al. Cationic polyelectrolytes, stable in 10 M KOHaq at 100 °C. ACS Macro Lett. 6, 1089–1093 (2017).
Ge, A. et al. Interfacial structure and electric field probed by in situ electrochemical vibrational Stark effect spectroscopy and computational modeling. J. Phys. Chem. C 121, 18674–18682 (2017).
Chen, L. D., Urushihara, M., Chan, K. & Nørskov, J. K. Electric field effects in electrochemical CO2 reduction. ACS Catal. 6, 7133–7139 (2016).
Harroun, S. G. et al. Electrochemical surface-enhanced Raman spectroscopy (E-SERS) of novel biodegradable ionic liquids. Phys. Chem. Chem. Phys. 15, 19205–19212 (2013).
Fu, J. Y. et al. In situ Raman monitoring of potential-dependent adlayer structures on the Au(111)/ionic liquid interface. Langmuir 38, 6209–6216 (2022).
Dunwell, M., Yan, Y. & Xu, B. Understanding the influence of the electrochemical double-layer on heterogeneous electrochemical reactions. Curr. Opin. Chem. Eng. 20, 151–158 (2018).
Malkani, A. S. et al. Understanding the electric and non-electric field components of the cation effect on the electrochemical CO reduction reaction. Sci. Adv. 6, eabd2569 (2020).
Salvatore, D. A. et al. Designing anion-exchange membranes for CO2 electrolysers. Nat. Energy 6, 339–348 (2021).
Deimede, V., Voyiatzis, G. A., Kallitsis, J. K., Qingfeng, L. & Bjerrum, N. J. Miscibility behavior of polybenzimidazole/sulfonated polysulfone blends for use in fuel cell applications. Macromolecules 33, 7609–7617 (2000).
Li, Q., He, R., Berg, R. W., Hjuler, H. A. & Bjerrum, N. J. Water uptake and acid doping of polybenzimidazoles as electrolyte membranes for fuel cells. Solid State Ion. 168, 177–185 (2004).
Quartarone, E. et al. Pyridine-based PBI composite membranes for PEMFCs. Fuel Cells 9, 349–355 (2009).
Chen, Z. Water balancing. Nat. Energy 5, 12–13 (2020).
Bonn, M. et al. Suppression of proton mobility by hydrophobic hydration. J. Am. Chem. Soc. 131, 17070–17071 (2009).
Zhang, C. et al. Water at hydrophobic interfaces delays proton surface-to-bulk transfer and provides a pathway for lateral proton diffusion. Proc. Natl Acad. Sci. USA 109, 9744–9749 (2012).
Liu, X. et al. pH effects on the electrochemical reduction of CO(2) towards C2 products on stepped copper. Nat. Commun. 10, 32 (2019).
Cao-Thang, D. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Hori, Y. et al. ‘Deactivation of copper electrode’ in electrochemical reduction of CO2. Electrochim. Acta 50, 5354–5369 (2005).
Miao, R. K. et al. Electroosmotic flow steers neutral products and enables concentrated ethanol electroproduction from CO2. Joule 5, 2742–2753 (2021).
O'Brien, C. P. et al. Single pass CO2 conversion exceeding 85% in the electrosynthesis of multi-carbon products via local CO2 regeneration. ACS Energy Lett. 6, 2952–2959 (2021).
Stern, O. Zur Theorie der elektrolytischen Doppelschicht. Z. Elektrochem. Angew. Physikal. Chem. 30, 508–516 (1924).
Thompson, A. P. et al. LAMMPS—a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 271, 108171 (2022).
Martinez, L., Andrade, R., Birgin, E. G. & Martinez, J. M. PACKMOL: a package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 30, 2157–2164 (2009).
Gaussian 16 Rev. C.01 (Gaussian, 2016).
Krishnan, R., Binkley, J. S., Seeger, R. & Pople, J. A. Self‐consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 72, 650–654 (1980).
Heinz, H., Vaia, R. A., Farmer, B. L. & Naik, R. R. Accurate simulation of surfaces and interfaces of face-centered cubic metals using 12–6 and 9–6 Lennard–Jones potentials. J. Phys. Chem. C 112, 17281–17290 (2008).
Joung, I. S. & Cheatham, T. E. III. Determination of alkali and halide monovalent ion parameters for use in explicitly solvated biomolecular simulations. J. Phys. Chem. B 112, 9020–9041 (2008).
Sun, C., Wen, B. & Bai, B. Application of nanoporous graphene membranes in natural gas processing: molecular simulations of CH4/CO2, CH4/H2S and CH4/N2 separation. Chem. Eng. Sci. 138, 616–621 (2015).
Jewett, A. I. et al. Moltemplate: a tool for coarse-grained modeling of complex biological matter and soft condensed matter physics. J. Mol. Biol. 433, 166841 (2021).
He, X., Man, V. H., Yang, W., Lee, T.-S. & Wang, J. A fast and high-quality charge model for the next generation general AMBER force field. J. Chem. Phys. 153, 114502 (2020).
Bayly, C. I., Cieplak, P., Cornell, W. & Kollman, P. A. A well-behaved electrostatic potential-based method using charge restraints for deriving atomic charges: the RESP model. J. Phys. Chem. 97, 10269–10280 (1993).
Tee, S. R. & Searles, D. J. Fully periodic, computationally efficient constant potential molecular dynamics simulations of ionic liquid supercapacitors. J. Chem. Phys. 156, 184101 (2022).
Ahrens-Iwers, L. J. V., Janssen, M., Tee, S. R. & Meissner, R. H. ELECTRODE: an electrochemistry package for atomistic simulations. J. Chem. Phys. 157, 084801 (2022).
Langford, L. et al. Constant-potential molecular dynamics simulations of molten-salt double layers for FLiBe and FLiNaK. J. Chem. Phys. 157, 094705 (2022).
Li, X. Y. et al. Molecular understanding of the Helmholtz capacitance difference between Cu(100) and graphene electrodes. J. Chem. Phys. 158, 084701 (2023).
Bussi, G., Donadio, D. & Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 126, 014101 (2007).
Michaud-Agrawal, N., Denning, E. J., Woolf, T. B. & Beckstein, O. MDAnalysis: a toolkit for the analysis of molecular dynamics simulations. J. Comput. Chem. 32, 2319–2327 (2011).
Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—the Open Visualization Tool. Model. Simul. Mat. Sci. Eng. 18, 015012 (2010).
Acknowledgements
We acknowledge support from the Natural Sciences and Engineering Research Council (NSERC) of Canada and TotalEnergies SE. Support from Canada Research Chairs Program is also gratefully acknowledged. Infrastructure provided through the Canada Foundation for Innovation and the Ontario Research Fund supported the work. R.K.M. thanks NSERC, Hatch and the Government of Ontario for their support through graduate scholarships. P.O. thanks the Climate Positive Energy for its support through Rising Stars in Clean Energy Postdoctoral Fellowship. Density functional theory calculations were performed on the Niagara supercomputer at the SciNet HPC Consortium. SciNet is funded by: the Canada Foundation for Innovation; the Government of Ontario; Ontario Research Fund - Research Excellence; and the University of Toronto. The computational study is supported by the Marsden Fund Council from Government funding (21-UOA-237) and Catalyst: Seeding General Grant (22-UOA-031-CGS), managed by Royal Society Te Apārangi. Z.W. and Y.M. acknowledge the use of New Zealand eScience Infrastructure (NeSI) high-performance computing facilities, consulting support and/or training services as part of this research. G.I.N.W. and Y.M. acknowledge funding support from the MacDiarmid Institute for Advanced Materials and Nanotechnology, the Energy Education Trust of New Zealand and the Royal Society Te Apārangi. Z.W. and Y.M. graciously acknowledge D. J. Searles (University of Queensland) and J. Cheng (Xiamen University) for their support and scientific discussions on the computational work.
Author information
Authors and Affiliations
Contributions
D.S. and E.H.S. supervised the project. M.F., J.E.H. and R.K.M. designed and carried out all the experiments. J.E.H. conceived the idea. Y.M., P.O. and Y.C. carried out the MD simulation. Z.W. supervised the MD simulation. Y.M., Y.C., G.I.N.W. and Z.W. analysed the MD simulation results. M.F. and J.E.H. analysed the experimental data and prepared the paper. R.K.M. performed the slim-flow cell design and experiments. F.L. carried out the COMSOL simulation. M.F. carried out all EIS measurements and analysed data. J.E.H. and Z.C. carried out the SERS measurements, and M.F. analysed the SERS data. Z.Z. performed the pH-sensitive Raman tests. J.Z., A.O. and Y.W. synthesized catalysts. W.N. and Y.Z. carried out scanning electron microscopy characterization. Y.Y. performed the X-ray diffraction characterization. B.K. and K.G. carried out the contact angle measurements. C.P.O., Y.X. and Y.C.X. performed product analysis. All authors discussed the results and assisted during paper preparation.
Corresponding authors
Ethics declarations
Competing interests
There is a US provisional patent application (63/381.180) titled ‘A modified catalyst for operating electrochemical carbon dioxide reduction in a non-alkali acidic medium and related techniques’, filed by the authors M.F., J.E.H., R.K.M., E.H.S. and D.S of this article and their institutions. The other authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Peng Kang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–7, Figs. 1–43, Tables 1–11and Videos 1–3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, M., Huang, J.E., Miao, R.K. et al. Cationic-group-functionalized electrocatalysts enable stable acidic CO2 electrolysis. Nat Catal 6, 763–772 (2023). https://doi.org/10.1038/s41929-023-01003-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01003-5
This article is cited by
-
Coverage enhancement accelerates acidic CO2 electrolysis at ampere-level current with high energy and carbon efficiencies
Nature Communications (2024)
-
Acidic media enables oxygen-tolerant electrosynthesis of multicarbon products from simulated flue gas
Nature Communications (2024)
-
Electrochemical Carbon Dioxide Reduction in Acidic Media
Electrochemical Energy Reviews (2024)
-
Immobilized cations boost acidic CO2 reduction
Nature Catalysis (2023)
-
Electrifying the future: the advances and opportunities of electrocatalytic carbon dioxide reduction in acid
Science China Chemistry (2023)