Abstract
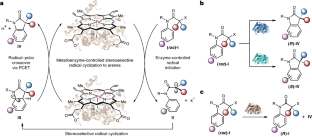
The effective induction of high levels of stereocontrol for free-radical-mediated transformations represents a notorious challenge in asymmetric catalysis. Herein, we describe a metalloredox biocatalysis strategy to repurpose natural cytochromes P450 to catalyse asymmetric radical cyclization to arenes through an unnatural electron transfer mechanism. Directed evolution afforded a series of engineered P450 aromatic radical cyclases with complementary selectivities: P450arc1 and P450arc2 facilitated enantioconvergent transformations of racemic substrates, giving rise to either enantiomer of the product with excellent total turnover numbers (up to 12,000). In addition to these enantioconvergent variants, another engineered radical cyclase, P450arc3, permitted efficient kinetic resolution of racemic chloride substrates (S factor = 18). Furthermore, computational studies revealed a proton-coupled electron transfer mechanism for the radical–polar crossover step, suggesting the potential role of the haem carboxylate as a base catalyst. Collectively, the excellent tunability of this metalloenzyme family provides an exciting platform for harnessing free radical intermediates for asymmetric catalysis.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are available in the main text and the Supplementary Information or available from the authors upon reasonable request. X-ray crystal structures of 2i and (R)-3a are available free of charge from the Cambridge Crystallographic Data Centre under reference numbers CCDC 2184585 and 2184586. Plasmids encoding P450arcs reported in this study are available for research purposes from Y.Y. under a material transfer agreement with the University of California Santa Barbara.
References
Bornscheuer, U. T. et al. Engineering the third wave of biocatalysis. Nature 485, 185–194 (2012).
Reetz, M. T. Laboratory evolution of stereoselective enzymes: a prolific source of catalysts for asymmetric reactions. Angew. Chem. Int. Ed. 50, 138–174 (2011).
Devine, P. N. et al. Extending the application of biocatalysis to meet the challenges of drug development. Nat. Rev. Chem. 2, 409–421 (2018).
Zetzsche, L. E., Chakrabarty, S. & Narayan, A. R. H. The transformative power of biocatalysis in convergent synthesis. J. Am. Chem. Soc. 144, 5214–5225 (2022).
Zetzsche, L. E. & Narayan, A. R. H. Broadening the scope of biocatalytic C–C bond formation. Nat. Rev. Chem. 4, 334–346 (2020).
Renata, H., Wang, Z. J. & Arnold, F. H. Expanding the enzyme universe: accessing non-natural reactions by mechanism-guided directed evolution. Angew. Chem. Int. Ed. 54, 3351–3367 (2015).
Leveson-Gower, R. B., Mayer, C. & Roelfes, G. The importance of catalytic promiscuity for enzyme design and evolution. Nat. Rev. Chem. 3, 687–705 (2019).
Chen, K. & Arnold, F. H. Engineering new catalytic activities in enzymes. Nat. Catal. 3, 203–213 (2020).
Miller, D. C., Athavale, S. V. & Arnold, F. H. Combining chemistry and protein engineering for new-to-nature biocatalysis. Nat. Synth. 1, 18–23 (2022).
Klaus, C. & Hammer, S. C. New catalytic reactions by enzyme engineering. Trends Chem. 4, 363–366 (2022).
Jäger, C. M. & Croft, A. K. Anaerobic radical enzymes for biotechnology. ChemBioEng Rev. 5, 143–162 (2018).
Broderick, J. B., Duffus, B. R., Duschene, K. S. & Shepard, E. M. Radical S-adenosylmethionine enzymes. Chem. Rev. 114, 4229–4317 (2014).
Sandoval, B. A. & Hyster, T. K. Emerging strategies for expanding the toolbox of enzymes in biocatalysis. Curr. Opin. Chem. Biol. 55, 45–51 (2020).
Harrison, W., Huang, X. & Zhao, H. Photobiocatalysis for abiological transformations. Acc. Chem. Res. 55, 1087–1096 (2022).
Emmanuel, M. A., Greenberg, N. R., Oblinsky, D. G. & Hyster, T. K. Accessing non-natural reactivity by irradiating nicotinamide-dependent enzymes with light. Nature 540, 414–417 (2016).
Sandoval, B. A., Meichan, A. J. & Hyster, T. K. Enantioselective hydrogen atom transfer: discovery of catalytic promiscuity in flavin-dependent ‘ene’-reductases. J. Am. Chem. Soc. 139, 11313–11316 (2017).
Biegasiewicz, K. F. et al. Photoexcitation of flavoenzymes enables a stereoselective radical cyclization. Science 364, 1166 (2019).
Black, M. J. et al. Asymmetric redox-neutral radical cyclization catalysed by flavin-dependent ‘ene’-reductases. Nat. Chem. 12, 71–75 (2020).
Page, C. G. et al. Quaternary charge-transfer complex enables photoenzymatic intermolecular hydroalkylation of olefins. J. Am. Chem. Soc. 143, 97–102 (2021).
Fu, H. et al. An asymmetric sp3–sp3 cross-electrophile coupling using ‘ene’-reductases. Nature 610, 302–307 (2022).
Huang, X. et al. Photoenzymatic enantioselective intermolecular radical hydroalkylation. Nature 584, 69–74 (2020).
Huang, X. et al. Photoinduced chemomimetic biocatalysis for enantioselective intermolecular radical conjugate addition. Nat. Catal. 5, 586–593 (2022).
Zhou, Q., Chin, M., Fu, Y., Liu, P. & Yang, Y. Stereodivergent atom-transfer radical cyclization by engineered cytochromes P450. Science 374, 1612–1616 (2021).
Fu, Y. et al. Engineered P450 atom-transfer radical cyclases are bifunctional biocatalysts: reaction mechanism and origin of enantioselectivity. J. Am. Chem. Soc. 144, 13344–13355 (2022).
Rui, J. et al. Directed evolution of nonheme iron enzymes to access abiological radical-relay C(sp3)−H azidation. Science 376, 869–874 (2022).
Proctor, R. S. J., Colgan, A. C. & Phipps, R. J. Exploiting attractive non-covalent interactions for the enantioselective catalysis of reactions involving radical intermediates. Nat. Chem. 12, 990–1004 (2020).
Mondal, S. et al. Enantioselective radical reactions using chiral catalysts. Chem. Rev. 122, 5842–5976 (2022).
Sibi, M. P., Manyem, S. & Zimmerman, J. Enantioselective radical processes. Chem. Rev. 103, 3263–3296 (2003).
Clark, A. J. Copper catalyzed atom transfer radical cyclization reactions. Eur. J. Org. Chem. 2016, 2231–2243 (2016).
Bhat, V., Welin, E. R., Guo, X. & Stoltz, B. M. Advances in stereoconvergent catalysis from 2005 to 2015: transition-metal-mediated stereoablative reactions, dynamic kinetic resolutions, and dynamic kinetic asymmetric transformations. Chem. Rev. 117, 4528–4561 (2017).
Keith, J. M., Larrow, J. F. & Jacobsen, E. N. Practical considerations in kinetic resolution reactions. Adv. Synth. Catal. 343, 5–26 (2001).
Vedejs, E. & Jure, M. Efficiency in nonenzymatic kinetic resolution. Angew. Chem. Int. Ed. 44, 3974–4001 (2005).
Zheng, Y., Zhang, S., Low, K.-H., Zi, W. & Huang, Z. A unified and desymmetric approach to chiral tertiary alkyl halides. J. Am. Chem. Soc. 144, 1951–1961 (2022).
Poulos, T. L. Heme enzyme structure and function. Chem. Rev. 114, 3919–3962 (2014).
Phillips, I. R., Shephard, E. A. & Ortiz de Montellano, P. R. Cytochrome P450 Protocols (Humana, 2013).
Brandenberg, O. F., Fasan, R. & Arnold, F. H. Exploiting and engineering hemoproteins for abiological carbene and nitrene transfer reactions. Curr. Opin. Biotechnol. 47, 102–111 (2017).
Yang, Y. & Arnold, F. H. Navigating the unnatural reaction space: directed evolution of heme proteins for selective carbene and nitrene transfer. Acc. Chem. Res. 54, 1209–1225 (2021).
Zhou, F., Liu, Y.-L. & Zhou, J. Catalytic asymmetric synthesis of oxindoles bearing a tetrasubstituted stereocenter at the C-3 position. Adv. Synth. Catal. 352, 1381–1407 (2010).
Whitehouse, C. J. C., Bell, S. G. & Wong, L.-L. P450BM3 (CYP102A1): connecting the dots. Chem. Soc. Rev. 41, 1218–1260 (2012).
Yang, Y., Cho, I., Qi, X., Liu, P. & Arnold, F. H. An enzymatic platform for the asymmetric amination of primary, secondary and tertiary C(sp3)–H bonds. Nat. Chem. 11, 987–993 (2019).
Mai, B. K., Neris, N. M., Yang, Y. & Liu, P. C–N bond forming radical rebound is the enantioselectivity-determining step in P411-catalyzed enantioselective C(sp3)–H amination: a combined computational and experimental investigation. J. Am. Chem. Soc. 144, 11215–11225 (2022).
Acevedo-Rocha, C. G., Hoebenreich, S. & Reetz, M. T. In Directed Evolution Library Creation: Methods and Protocols (eds. Gillam, E. M. J. et al.) 103–128 (Springer, 2014).
Weinberg, D. R. et al. Proton-coupled electron transfer. Chem. Rev. 112, 4016–4093 (2012).
Warren, J. J. & Mayer, J. M. Proton-coupled electron transfer reactions at a heme-propionate in an iron-protoporphyrin-IX model compound. J. Am. Chem. Soc. 133, 8544–8551 (2011).
Acknowledgements
This research is supported by the NIH (R35GM147387 to Y.Y. and R35GM128779 to P.L.) and the University of California Santa Barbara (Y.Y.). We acknowledge the BioPACIFIC MIP (NSF Materials Innovation Platform, DMR-1933487) and the NSF MRSEC Program (DMR-1720256) for access to instrumentation. DFT calculations were performed at the Center for Research Computing of the University of Pittsburgh and the Extreme Science and Engineering Discovery Environment supported by the National Science Foundation grant number ACI-1548562. Y.F. is an Andrew W. Mellon Predoctoral Fellow. We thank L. Zhang (University of California Santa Barbara) and Y. Wang (University of Pittsburgh) for critical reading of this manuscript.
Author information
Authors and Affiliations
Contributions
Y.Y. conceived and directed the project. W.F., N.M.N., Y.Z. and B.K.-H. designed and performed the experiments. Y.F. carried out the computational studies with P.L. providing guidance. Y.Y., Y.F. and P.L. wrote the manuscript with the input of all other authors.
Corresponding authors
Ethics declarations
Competing interests
Y.Y., W.F., N.M.N. and Y.Z. are inventors on a patent application (US provisional patent no. 63/477,081) submitted by the University of California Santa Barbara that covers stereoselective biocatalytic radical addition to arenes. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Gonzalo Jiménez-Osé and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Tables 1–15, Figs. 1–26, Notes 1–12, Discussion and References.
Supplementary Data 1
Crystallographic data for compound 2i.
Supplementary Data 2
Crystallographic data for compound 3a.
Supplementary Data 3
Atomic coordinates of the optimized computational models from molecular dynamics simulations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, W., Neris, N.M., Fu, Y. et al. Enzyme-controlled stereoselective radical cyclization to arenes enabled by metalloredox biocatalysis. Nat Catal 6, 628–636 (2023). https://doi.org/10.1038/s41929-023-00986-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-00986-5