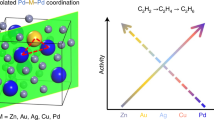
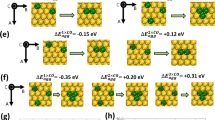
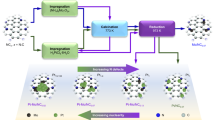
Abstract
Connecting active-site chemistry with observed macroscopic kinetic behaviour is required to rationally design active sites of heterogeneous catalysts. Isolated active sites limit co-adsorption complexities, which challenge reconciling elementary reaction mechanisms and rate constants to observed macroscopic kinetics. The Pd–Zn γ-brass intermetallic phase enables the controlled synthesis of Pd1 monomer and Pd3 trimer sites isolated in an inert Zn matrix. Here we utilize these isolated sites, combining experimental kinetic measurements, density functional theory (DFT) calculations and a fully coverage-enumerated microkinetic model (MKM) to provide detailed mechanistic understanding of elementary reaction chemistry for ethylene hydrogenation. With isolated sites reducing the complexity of co-adsorption coverage effects, remarkable agreement between experimental and DFT-MKM kinetics is reached. The acute temperature dependence of reaction orders, the site competition between C2 species and hydrogen, the degree of rate control of elementary reactions and the steady-state distribution of co-adsorption configurations are reconciled.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data presented in the main figures of the manuscript and all structure files (in VASP CONTCAR format) are available at https://github.com/mjjanik/NatCatal2023. Source data are provided with this paper.
Code availability
Mathematical notebooks used to perform microkinetic models are available at https://github.com/mjjanik/NatCatal2023.
References
Horiuti, I. & Polanyi, M. Exchange reactions of hydrogen on metallic catalysts. Trans. Faraday Soc. 30, 1164–1172 (1934).
Rekoske, J. E., Cortright, R. D., Goddard, S. A., Sharma, S. B. & Dumesic, J. A. Microkinetic analysis of diverse experimental data for ethylene hydrogenation on platinum. J. Phys. Chem. 96, 1880–1888 (2002).
Goddard, S. Deuterium tracing studies and microkinetic analysis of ethylene hydrogenation over platinum. J. Catal. 137, 186–198 (1992).
Heard, C. J., Hu, C., Skoglundh, M., Creaser, D. & Grönbeck, H. Kinetic regimes in ethylene hydrogenation over transition-metal surfaces. ACS Catal. 6, 3277–3286 (2016).
Cortright, R. D., Goddard, S. A., Rekoske, J. E. & Dumesic, J. A. Kinetic study of ethylene hydrogenation. J. Catal. 127, 342–353 (1991).
Boudart, M. Two-step catalytic reactions. AIChE J. 18, 465–478 (1972).
Grabow, L. C., Gokhale, A. A., Evans, S. T., Dumesic, J. A. & Mavrikakis, M. Mechanism of the water gas shift reaction on Pt: first principles, experiments, and microkinetic modeling. J. Phys. Chem. C 112, 4608–4617 (2008).
Zhao, J., Zha, S., Mu, R., Zhao, Z.-J. & Gong, J. Coverage effect on the activity of the acetylene semihydrogenation over Pd–Sn catalysts: a density functional theory study. J. Phys. Chem. C 122, 6005–6013 (2018).
Palermo, A. et al. Dialing in single-site reactivity of a supported calixarene-protected tetrairidium cluster catalyst. Chem. Sci. 8, 4951–4960 (2017).
Kuo, C.-T. et al. 18.1% single palladium atom catalysts on mesoporous covalent organic framework for gas phase hydrogenation of ethylene. Cell Rep. Phys. Sci. https://doi.org/10.1016/j.xcrp.2021.100495 (2021).
Dasgupta, A. et al. Atomic control of active-site ensembles in ordered alloys to enhance hydrogenation selectivity. Nat. Chem. 14, 523–529 (2022).
Prinz, J. et al. Adsorption of small hydrocarbons on the three-fold PdGa surfaces: the road to selective hydrogenation. J. Am. Chem. Soc. 136, 11792–11798 (2014).
Cremer, P. S., Su, X., Shen, Y. R. & Somorjai, G. A. Ethylene hydrogenation on Pt(111) monitored in situ at high pressures using sum frequency generation. J. Am. Chem. Soc. 118, 2942–2949 (1996).
Zaera, F. On the mechanism for the hydrogenation of olefins on transition-metal surfaces: the chemistry of ethylene on Pt(111). Langmuir 12, 88–94 (1996).
Beebe, T. P. & Yates, J. T. An in situ infrared spectroscopic investigation of the role of ethylidyne in the ethylene hydrogenation reaction on palladium/alumina. J. Am. Chem. Soc. 108, 663–671 (2002).
Cremer, P. S. & Somorjai, G. A. Surface science and catalysis of ethylene hydrogenation. J. Chem. Soc. Faraday Trans. 91, 3671–3677 (1995).
Zaera, F. & Somorjai, G. A. Hydrogenation of ethylene over platinum (111) single-crystal surfaces. J. Am. Chem. Soc. 106, 2288–2293 (2002).
Godbey, D., Zaera, F., Yeates, R. & Somorjai, G. A. Hydrogenation of chemisorbed ethylene on clean, hydrogen, and ethylidyne covered platinum (111) crystal surfaces. Surf. Sci. 167, 150–166 (1986).
Moskaleva, L. V. et al. Ethylene conversion to ethylidyne over Pd(111): revisiting the mechanism with first-principles calculations. J. Phys. Chem. C 113, 2512–2520 (2009).
Sutton, J. E., Guo, W., Katsoulakis, M. A. & Vlachos, D. G. Effects of correlated parameters and uncertainty in electronic-structure-based chemical kinetic modelling. Nat. Chem. 8, 331–337 (2016).
Wellendorff, J. et al. A benchmark database for adsorption bond energies to transition metal surfaces and comparison to selected DFT functionals. Surf. Sci. 640, 36–44 (2015).
Matera, S., Schneider, W. F., Heyden, A. & Savara, A. Progress in accurate chemical kinetic modeling, simulations, and parameter estimation for heterogeneous catalysis. ACS Catal. 9, 6624–6647 (2019).
Stegelmann, C., Andreasen, A. & Campbell, C. T. Degree of rate control: how much the energies of intermediates and transition states control rates. J. Am. Chem. Soc. 131, 8077–8082 (2009).
Campbell, C. T. The degree of rate control: a powerful tool for catalysis research. ACS Catal. 7, 2770–2779 (2017).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 78, 1396–1396 (1997).
Methfessel, M. & Paxton, A. T. High-precision sampling for Brillouin-zone integration in metals. Phys. Rev. B 40, 3616–3621 (1989).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Henkelman, G. & Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978–9985 (2000).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Campbell, C. T. & Sellers, J. R. The entropies of adsorbed molecules. J. Am. Chem. Soc. 134, 18109–18115 (2012).
Bradley, A. J. & Thewlis, J. The structure of γ-brass. Proc. R. Soc. Lond. A 112, 678–692 (1997).
Edström, V.-A. et al. X-ray determination of the structure of the cubic gamma Pd,Zn phase. Acta Chem. Scand. 23, 279–285 (1969).
Spanjers, C. S. et al. Determination of bulk and surface atomic arrangement in Ni–Zn γ-brass phase at different Ni to Zn ratios. Chem. Mater. 29, 504–512 (2016).
Gourdon, O. et al. Atomic distributions in the γ-brass structure of the Cu–Zn system: a structural and theoretical study. Inorg. Chem. 46, 251–260 (2007).
Larson, A. C. & Von Dreele, R. B. General Structure Analysis System (GSAS) Report LAUR 86-748 (Los Alamos National Laboratory, 2004).
Acknowledgements
This work is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, Catalysis Division under award no. DE-SC0020147. This work used the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by the National Science Foundation under grant no. ACI-1548562.
Author information
Authors and Affiliations
Contributions
H.H. and A.N. carried out and analysed the DFT calculations, microkinetic models, developed genetic algorithms and performed degree of rate control analysis. G.C. and A.D. synthesized the materials, performed the materials characterization and measured reaction kinetics. H.H. and G.C. wrote the manuscript. A.N. and R.J.M. helped with editing the paper. M.J.J. and R.M.R. supervised the project and established the final version of the manuscript. All authors contributed to the manuscript and have approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Shinya Furukawa, Simon Beaumont and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–18, Figs. 1–18 and Table 1.
Source data
Source Data Fig. 1
Source data in Excel workbook.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, H., Canning, G.A., Nguyen, A. et al. Active-site isolation in intermetallics enables precise identification of elementary reaction kinetics during olefin hydrogenation. Nat Catal 6, 596–605 (2023). https://doi.org/10.1038/s41929-023-00978-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-00978-5