Abstract
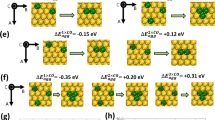
Intermetallic compounds offer unique opportunities for atom-by-atom manipulation of catalytic ensembles through precise stoichiometric control. The (Pd, M, Zn) γ-brass phase enables the controlled synthesis of Pd–M–Pd catalytic sites (M = Zn, Pd, Cu, Ag and Au) isolated in an inert Zn matrix. These multi-atom heteronuclear active sites are catalytically distinct from Pd single atoms and fully coordinated Pd. Here we quantify the unexpectedly large effect that active-site composition (that is, identity of the M atom in Pd–M–Pd sites) has on ethylene selectivity during acetylene semihydrogenation. Subtle stoichiometric control demonstrates that Pd–Pd–Pd sites are active for ethylene hydrogenation, whereas Pd–Zn–Pd sites show no measurable ethylene-to-ethane conversion. Agreement between experimental and density-functional-theory-predicted activities and selectivities demonstrates precise control of Pd–M–Pd active-site composition. This work demonstrates that the diversity and well-defined structure of intermetallics can be used to design active sites assembled with atomic-level precision.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information files. Source data are provided with this paper.
Code availability
All computational codes/algorithms utilized in this study were performed with either commercially available or open-source software.
References
Norskov, J. K., Bligaard, T., Rossmeisl, J. & Christensen, C. H. Towards the computational design of solid catalysts. Nat. Chem. 1, 37–46 (2009).
Besenbacher, F. et al. Design of a surface alloy catalyst for steam reforming. Science 279, 1913–1915 (1998).
Paffett, M. T., Gebhard, S. C., Windham, R. G. & Koel, B. E. Chemisorption of ethylene on ordered Sn/Pt(111) surface alloys. Surf. Sci. 223, 449–464 (1989).
Rodriguez, J. A. Interactions in bimetallic bonding: electronic and chemical properties of PdZn surfaces. J. Phys. Chem. 98, 5758–5764 (1994).
Weilach, C. et al. Geometric arrangement of components in bimetallic PdZn/Pd(111) surfaces modified by CO adsorption: a combined study by density functional calculations, polarization-modulated infrared reflection absorption spectroscopy, and temperature-programmed desorption. J. Phys. Chem. C 116, 18768–18778 (2012).
Chen, M. S., Kumar, D., Yi, C. W. & Goodman, D. W. The promotional effect of gold in catalysis by palladium-gold. Science 310, 291–293 (2005).
Kyriakou, G. et al. Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations. Science 335, 1209–1212 (2012).
Zhang, S. R. et al. Catalysis on singly dispersed bimetallic sites. Nat. Commun. 6, 7938 (2015).
Qiao, B. T. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Spezzati, G. et al. Atomically dispersed Pd-O species on CeO2(111) as highly active sites for low-temperature CO oxidation. ACS Catal. 7, 6887–6891 (2017).
Wei, H. S. et al. FeOx-supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes. Nat. Commun. 5, 5634 (2014).
Furukawa, S. & Komatsu, T. Intermetallic compounds: promising inorganic materials for well-structured and electronically modified reaction environments for efficient catalysis. ACS Catal. 7, 735–765 (2017).
Kovnir, K. et al. A new approach to well-defined, stable and site-isolated catalysts. Sci. Technol. Adv. Mater. 8, 420–427 (2007).
Prinz, J. et al. Isolated Pd sites on the intermetallic PdGa(111) and PdGa(\(\bar 1\bar 1\bar 1\)) model catalyst surfaces. Angew. Chem. Int. Ed. 51, 9339–9343 (2012).
Osswald, J. et al. Palladium–gallium intermetallic compounds for the selective hydrogenation of acetylene: part II: surface characterization and catalytic performance. J. Catal. 258, 219–227 (2008).
Armbrüster, M. et al. Pd–Ga intermetallic compounds as highly selective semihydrogenation catalysts. J. Am. Chem. Soc. 132, 14745–14747 (2010).
Matselko, O. et al. Revealing electronic influences in semihydrogenation of acetylene. J. Phys. Chem. C 122, 21891–21896 (2018).
Luo, Y. et al. Addressing electronic effects in the semi-hydrogenation of ethyne by InPd2 and intermetallic Ga–Pd compounds. J. Catal. 338, 265–272 (2016).
Osswald, J. et al. Palladium–gallium intermetallic compounds for the selective hydrogenation of acetylene: part I: preparation and structural investigation under reaction conditions. J. Catal. 258, 210–218 (2008).
Li, C. M. et al. Nickel-gallium intermetallic nanocrystal catalysts in the semihydrogenation of phenylacetylene. ChemCatChem 6, 824–831 (2014).
Zhou, H. R. et al. PdZn intermetallic nanostructure with Pd–Zn–Pd ensembles for highly active and chemoselective semi-hydrogenation of acetylene. ACS Catal. 6, 1054–1061 (2016).
Feng, Q. C. et al. Isolated single-atom Pd sites in intermetallic nanostructures: high catalytic selectivity for semihydrogenation of alkynes. J. Am. Chem. Soc. 139, 7294–7301 (2017).
Haubrich, J. et al. Adsorption of α,β-unsaturated aldehydes on Pt(111) and Pt–Sn alloys: II. Crotonaldehyde. J. Phys. Chem. C 113, 13947–13967 (2009).
Prinz, J. et al. Adsorption of small hydrocarbons on the three-fold PdGa surfaces: the road to selective hydrogenation. J. Am. Chem. Soc. 136, 11792–11798 (2014).
Bradley, A. J. & Thewlis, J. The structure of γ-brass. Proc. R. Soc. Lond. A 112, 678–692 (1926).
Studt, F. et al. Identification of non-precious metal alloy catalysts for selective hydrogenation of acetylene. Science 320, 1320–1322 (2008).
Edstrom, V. A. & Westman, S. X-ray determination of structure of cubic gamma Pd,Zn phase. Acta Chem. Scand. 23, 279–285 (1969).
Connolly, J. W. D. & Williams, A. R. Density-functional theory applied to phase transformations in transition-metal alloys. Phys. Rev. B 27, 5169–5172 (1983).
Spanjers, C. S. et al. Determination of bulk and surface atomic arrangement in Ni–Zn γ-brass phase at different Ni to Zn ratios. Chem. Mater. 29, 504–512 (2017).
Huang, F. et al. Atomically dispersed Pd on nanodiamond/graphene hybrid for selective hydrogenation of acetylene. J. Am. Chem. Soc. 140, 13142–13146 (2018).
Gourdon, O. et al. Atomic distributions in the γ-brass structure of the Cu–Zn system: a structural and theoretical study. Inorg. Chem. 46, 251–260 (2007).
Spanjers, C. S. et al. Zinc inclusion to heterogeneous nickel catalysts reduces oligomerization during the semi-hydrogenation of acetylene. J. Catal. 316, 164–173 (2014).
Vonheidenstam, O., Johansson, A. & Westman, S. A redetermination of distribution of atoms in Cu5Zn8, Cu5Cd8 and Cu9Al4. Acta Chem. Scand. 22, 653–661 (1968).
Larson, A. C. & Dreele, R. B. V. General Structure Analysis System (GSAS) Report no. LAUR 86-748 (Los Alamos National Laboratory, 2000).
Toby, B. H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 34, 210–213 (2001).
Kresse, G. & Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Methfessel, M. & Paxton, A. T. High precision sampling for Brillouin zone integration in metals. Phys. Rev. B 40, 3616–3621 (1989).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Henkelman, G. & Jonsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978–9985 (2000).
Acknowledgements
This work is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, Catalysis Division under award no. DE-SC0020147. A.D. acknowledges financial support from the US National Science Foundation (grant no. CBET–1748365). H.H. acknowledges training provided by the Computational Materials Education and Training (CoMET) National Science Foundation Research Traineeship (grant no. DGE-1449785). This work used the Extreme Science and Engineering Discovery Environment, which is supported by the National Science Foundation under grant no. ACI-1548562.
Author information
Authors and Affiliations
Contributions
A.D., E.K.Z. and R.M.R. planned, executed and analysed the experimental work. H.H. and M.J.J. performed and analysed the DFT computational work. R.G., S.-L.S. and Z.-K.L. performed and analysed the CEM work. R.J.M., M.J.J. and R.M.R. conceived the work and supervised the research. A.D. and H.H. wrote the paper with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Siris Laursen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Projected density of states on surface Pd atoms.
Projected density of state of (a) surface Pd atoms of 3 × 3 5-layer Pd(111) slab with a d-band center of −1.36 eV, (b) surface Pd atoms of Pd8Zn44, with d-band center of −2.02 eV, (c) edge Pd of a Pd trimer site on the surface of Pd9Zn43, with d-band center of −1.96 eV (d) middle Pd of a Pd trimer site on the surface of Pd9Zn43, with d-band center of −1.99 eV (energies relative to Fermi levels).
Extended Data Fig. 2 SEM image of γ-Pd9Zn43 after re-annealing post ball milling at 500°C for 7 days.
The SEM image clearly shows particles have a platelet like morphology and a particle size ranging from 1 to ~10 micron.
Supplementary information
Supplementary Information
Supplementary Figs. 1–16, Tables 1–9 and Sections 1–9 providing additional information on methods and discussion of results.
Supplementary Data 1
A compressed zip file containing all optimized DFT structures (in Vienna Ab initio Simulation Package CONTCAR format).
Supplementary Data 2
A compressed zip file containing all structures, parameters and data for the CEM analysis.
Source data
Source Data Fig. 1
Excel file containing all data that appear in graphs within Fig. 1.
Source Data Fig. 2
Excel file containing all data that appear in graphs within Fig. 2.
Source Data Fig. 3
Excel file containing all data that appear in graphs within Fig. 3.
Source Data Fig. 4
Excel file containing all data that appear in graphs within Fig. 4.
Source Data Fig. 5
Excel file containing all data that appear in graphs within Fig. 5.
Source Data Extended Data Fig. 1
Excel file containing all data that appear in graphs within Extended Data Fig. 1.
Rights and permissions
About this article
Cite this article
Dasgupta, A., He, H., Gong, R. et al. Atomic control of active-site ensembles in ordered alloys to enhance hydrogenation selectivity. Nat. Chem. 14, 523–529 (2022). https://doi.org/10.1038/s41557-021-00855-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00855-3
This article is cited by
-
A MOF-supported Pd1–Au1 dimer catalyses the semihydrogenation reaction of acetylene in ethylene with a nearly barrierless activation energy
Nature Catalysis (2024)
-
Fundamentals and catalytic applications of single-atom alloys
Science China Materials (2024)
-
Tripodal Pd metallenes mediated by Nb2C MXenes for boosting alkynes semihydrogenation
Nature Communications (2023)
-
Active-site isolation in intermetallics enables precise identification of elementary reaction kinetics during olefin hydrogenation
Nature Catalysis (2023)
-
Tunable hydrogen coverage on electron-deficient platinum nanoparticles for efficient hydrogenation reactions
Nano Research (2023)