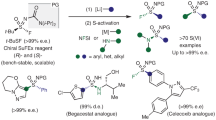
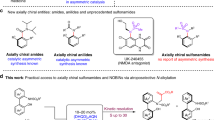
Abstract
Enantioenriched sulfoxides have found ever-increasing applications in marketed drugs or as candidates in clinical trials. They are also frequently used as key motifs in chiral ligands or as organocatalysts in asymmetric catalysis. However, the asymmetric synthesis of such a functionality poses a significant challenge. Here, by harnessing the strong backbonding donation of Ni(0) to alkynes, we report a Ni-catalysed hydrosulfenation reaction of unactivated alkynes with in situ-generated sulfenic acids, enabling the reaction to proceed in an enantioselective stepwise mechanism that is contrary to the well-established uncatalysed concerted mechanism. A plethora of alkenyl sulfoxides were synthesized with high enantioselectivity and a broad substrate scope. The products could be easily elaborated into structurally diverse sulfoxides that could serve as chiral ligands, catalysts and useful pharmacophores for the late-stage modification of drugs.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All information relating to optimization studies, experimental procedures, mechanistic studies, DFT calculations, high-performance liquid chromatography spectra, NMR spectra, high-resolution mass spectrometry and optical rotation data are available in Supplementary Information. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2117922 (3g), CCDC 2178299 (7) and CCDC 2124800 (8′). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other data are available from the corresponding authors upon request.
References
Gupta, V. & Carroll, K. S. Sulfenic acid chemistry, detection and cellular lifetime. Biochim. Biophys. Acta 1840, 847–875 (2014).
Carreno, M. C. Applications of sulfoxides to asymmetric synthesis of biologically active compounds. Chem. Rev. 95, 1717–1760 (1995).
Fernández, I. & Khiar, N. Recent developments in the synthesis and utilization of chiral sulfoxides. Chem. Rev. 103, 3651–3706 (2003).
Wojaczynska, E. & Wojaczynski, J. Modern stereoselective synthesis of chiral sulfinyl compounds. Chem. Rev. 120, 4578–4611 (2020).
Robak, M. T., Herbage, M. A. & Ellman, J. A. Synthesis and applications of tert-butanesulfinamide. Chem. Rev. 110, 3600–3740 (2010).
Trost, B. M. & Rao, M. Development of chiral sulfoxide ligands for asymmetric catalysis. Angew. Chem. Int. Ed. 54, 5026–5043 (2015).
Sipos, G., Drinkel, E. E. & Dorta, R. The emergence of sulfoxides as efficient ligands in transition metal catalysis. Chem. Soc. Rev. 44, 3834–3860 (2015).
Otocka, S., Kwiatkowska, M., Madalinska, L. & Kielbasinski, P. Chiral organosulfur ligands/catalysts with a stereogenic sulfur atom: applications in asymmetric synthesis. Chem. Rev. 117, 4147–4181 (2017).
Pitchen, P., Dunach, E., Deshmukh, M. N. & Kagan, H. B. An efficient asymmetric oxidation of sulfides to sulfoxides. J. Am. Chem. Soc. 106, 8188–8193 (1984).
Ma, L.-j et al. Chiral Brønsted-acid-catalyzed asymmetric oxidation of sulfenamide by using H2O2: a versatile access to sulfinamide and sulfoxide with high enantioselectivity. ACS Catal. 9, 1525–1530 (2019).
Maitro, G. et al. Enantioselective synthesis of aryl sulfoxides via palladium-catalyzed arylation of sulfenate anions. Org. Lett. 9, 5493–5496 (2007).
Zong, L., Ban, X., Kee, C. W. & Tan, C. H. Catalytic enantioselective alkylation of sulfenate anions to chiral heterocyclic sulfoxides using halogenated pentanidium salts. Angew. Chem. Int. Ed. 53, 11849–11853 (2014).
Jia, T. et al. Palladium-catalyzed enantioselective arylation of aryl sulfenate anions: a combined experimental and computational study. J. Am. Chem. Soc. 139, 8337–8345 (2017).
Wang, L., Chen, M., Zhang, P., Li, W. & Zhang, J. Palladium/PC-Phos-catalyzed enantioselective arylation of general sulfenate anions: scope and synthetic applications. J. Am. Chem. Soc. 140, 3467–3473 (2018).
Wu, C., Berritt, S., Liang, X. & Walsh, P. J. Palladium-catalyzed enantioselective alkenylation of sulfenate anions. Org. Lett. 21, 960–964 (2019).
Andersen, K. K. Synthesis of (+)-ethyl p-tolyl sulfoxide from (−)-menthyl (−)-p-toluenesulfinate. Tetrahedron Lett. 3, 93–95 (1962).
Andersen, K. K., Gaffield, W., Papanikolaou, N. E., Foley, J. W. & Perkins, R. I. Optically active sulfoxides. The synthesis and rotatory dispersion of some diaryl sulfoxides. J. Am. Chem. Soc. 86, 5637–5646 (1964).
Han, Z. et al. A highly selective and practical method for enantiopure sulfoxides utilizing activated and functionally differentiated N-sulfonyl-1,2,3-oxathiazolidine-2-oxide derivatives. Angew. Chem. Int. Ed. 42, 2032–2035 (2003).
Han, Z. et al. Development of a preparative-scale asymmetric synthesis of (R)-p-tolyl methyl sulfoxide for use in a one-pot synthesis of a drug intermediate containing a trifluoromethyl-substituted alcohol functionality. Org. Process Res. Dev. 11, 605–608 (2007).
Zhang, X., Ang, E. C. X., Yang, Z., Kee, C. W. & Tan, C. H. Synthesis of chiral sulfinate esters by asymmetric condensation. Nature 604, 298–303 (2022).
Shelton, J. R. & Davis, K. E. t-Butylsulfenic acid. J. Am. Chem. Soc. 89, 718–719 (1967).
Allison, W. S. Formation and reactions of sulfenic acids in proteins. Acc. Chem. Res. 9, 293–299 (1976).
Neville Jones, D., Cottam, P. D. & Davies, J. Addition of sulphenic acids to unactivated alkynes to give alkenyl sulphoxides. Tetrahedron Lett. 20, 4977–4980 (1979).
Guihaumé, J., Halbert, S., Eisenstein, O. & Perutz, R. N. Hydrofluoroarylation of alkynes with Ni Catalysts. C–H activation via ligand-to-ligand hydrogen transfer, an alternative to oxidative addition. Organometallics 31, 1300–1314 (2011).
Zhang, Y.-Q. et al. Ni-catalyzed asymmetric hydrophosphinylation of conjugated enynes and mechanistic studies. Chem. Sci. 13, 4095–4102 (2022).
Chatt, J. & Duncanson, L. A. 586. Olefin co-ordination compounds. Part III. Infra-red spectra and structure: attempted preparation of acetylene complexes. J. Chem. Soc. 2939–2947 (1953).
Chatt, J., Duncanson, L. A. & Venanzi, L. M. Directing effects in inorganic substitution reactions. Part I. A hypothesis to explain the trans effect. J. Chem. Soc. 4456–4460 (1955).
Allen, S. R., Green, M., Orpen, A. G. & Williams, I. D. The role of metallacyclopropenes in the formation of alkylidyne complexes; evidence for a 1,2-trimethylsilyl shift and the X-ray crystal structure of [Mo=C(SiMe3)CH2{P(OMe)3}2(η5-C9H7)]. J. Chem. Soc. Chem. Commun. 826–828 (1982).
Boatz, J. A., Gordon, M. S. & Sita, L. R. Theoretical studies of the metallacyclopropenes c-[MX2C2H2] (M = C, Si, Ge, Sn; X = H, F). J. Phys. Chem. 94, 5488–5493 (1990).
Edelbach, B. L., Lachicotte, R. J. & Jones, W. D. Catalytic carbon−carbon bond activation and functionalization by nickel complexes. Organometallics 18, 4040–4049 (1999).
Jemmis, E. D. et al. Are metallocene−acetylene (M = Ti, Zr, Hf) complexes aromatic metallacyclopropenes? Organometallics 29, 76–81 (2009).
Tasker, S. Z., Standley, E. A. & Jamison, T. F. Recent advances in homogeneous nickel catalysis. Nature 509, 299–309 (2014).
Bakewell, C., White, A. J. P. & Crimmin, M. R. Reversible alkene binding and allylic C-H activation with an aluminium(I) complex. Chem. Sci. 10, 2452–2458 (2019).
Wheeler, O. W., Coates, R. A., Lapoutre, V. J. F., Bakker, J. M. & Armentrout, P. B. Metallacyclopropene structures identified by IRMPD spectroscopic investigation of the dehydrogenation reactions of Ta+ and TaO+ with ethene. Int. J. Mass Spectrom. 442, 83–94 (2019).
Lv, Z. J., Chai, Z., Zhu, M., Wei, J. & Zhang, W. X. Selective coupling of lanthanide metallacyclopropenes and nitriles via azametallacyclopentadiene and η2-pyrimidine metallacycle. J. Am. Chem. Soc. 143, 9151–9161 (2021).
He, Q. et al. Palladium-catalyzed modular and enantioselective cis-difunctionalization of 1,3-enynes with imines and boronic reagents. J. Am. Chem. Soc. 143, 17989–17994 (2021).
Standley, E. A., Tasker, S. Z., Jensen, K. L. & Jamison, T. F. Nickel catalysis: synergy between method development and total synthesis. Acc. Chem. Res. 48, 1503–1514 (2015).
Xiao, L. J. et al. Nickel(0)-catalyzed hydroalkenylation of imines with styrene and its derivatives. Angew. Chem. Int. Ed. 57, 3396–3400 (2018).
Montgomery, J. Nickel-catalyzed cyclizations, couplings, and cycloadditions involving three reactive components. Acc. Chem. Res. 33, 467–473 (2000).
Orsino, A. F., Gutierrez Del Campo, M., Lutz, M. & Moret, M. E. Enhanced catalytic activity of nickel complexes of an adaptive diphosphine–benzophenoneligand in alkyne cyclotrimerization. ACS Catal. 9, 2458–2481 (2019).
Hao, J., Mougang-Soume, B., Vabre, B. & Zargarian, D. On the stability of a POCsp3OP-type pincer ligand in nickel(II) complexes. Angew. Chem. Int. Ed. 53, 3218–3222 (2014).
Linden, A. et al. ‘Chiral-at-metal’ hemilabile nickel complexes with a latent d10-ML2 configuration: receiving substrates with open arms. Organometallics 31, 6162–6171 (2012).
Bonrath, B. W., Porschke, K. R., Wilke, C., Angermund, K. & Kruger, C. Mono- and dinuclear nickel(0) complexes of butadiyne. Angew. Chem. Int. Ed. 27, 833–835 (1988).
Jiang, J., Fu, M., Li, C., Shang, R. & Fu, Y. Theoretical investigation on nickel-catalyzed hydrocarboxylation of alkynes employing formic acid. Organometallics 36, 2818–2825 (2017).
Ren, X., Lu, Y., Lu, G. & Wang, Z.-X. Density functional theory mechanistic study of Ni-catalyzed reductive alkyne-alkyne cyclodimerization: oxidative cyclization versus outer-sphere proton transfer. Org. Lett. 22, 2454–2459 (2020).
Acknowledgements
We are grateful for financial support from NSFC (22071224 and 21901235 to Q.-W.Z. and 21973055 to G.L.) and USTC Research Funds of the Double First-Class Initiative (YD2060002010 to Q.-W.Z.). We acknowledge the Taishan Scholar of Shandong Province (No. tsqn201812013 to G.L.) and the Qilu Young Scholar of Shandong University (to G.L.). The numerical calculations in this paper were performed on the supercomputing system in the Supercomputing Center of the University of Science and Technology of China and the HPC Cloud Platform of Shandong University.
Author information
Authors and Affiliations
Contributions
Q.-W.Z. conceived and supervised the project. Y.-Q.Z. performed the experiments and analysed the data. L.Y. carried out the synthesis of sulfoxide starting materials and collected the data. Q.-W.Z. and L.H. carried out the DFT calculations. Q.-W.Z. and G.L. co-wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–4, Figs. 1–9, Methods, copies of NMR and high-performance liquid chromatography data and References.
Supplementary Data 1
cif file of 3g.
Supplementary Data 2
cif file of 7.
Supplementary Data 3
cif file of 8′.
Supplementary Data 4
Cartesian coordinates of optimized structures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, YQ., Hu, L., Yuwen, L. et al. Nickel-catalysed enantioselective hydrosulfenation of alkynes. Nat Catal 6, 487–494 (2023). https://doi.org/10.1038/s41929-023-00966-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-00966-9