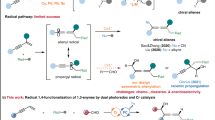
Abstract
Precise control over the selectivity of a reaction is a fundamental target. While great advances have been obtained in achieving stereocontrol, the selective manipulation of functional groups within a substrate (chemoselectivity) is still a challenge. The cyanation of aldehydes offers an illustrative example: the 1,2-addition of nucleophilic cyanide to the aldehydic group was one of the first examples of a stereoselective catalytic process. By contrast, the conjugate cyanation of linear α,β-unsaturated aldehydes has remained elusive, even in a racemic variant. The main difficulty lies in achieving 1,4-chemoselectivity over the preferred cyanide 1,2-addition. Here, we report an asymmetric catalytic method to achieve the exclusive conjugate cyanation of enals. The synergistic action of a chiral organocatalyst with a visible-light-activated photoredox catalyst promotes the single-electron reduction of enals, inducing a formal inversion of polarity. The resulting chiral radical, being nucleophilic in character, is then intercepted by an electrophilic cyanide source with perfect 1,4-chemoselectivity and good stereocontrol.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Materials and methods, experimental procedures, useful information, mechanistic studies, 1H NMR spectra, 13C NMR spectra and mass spectrometry data are available in Supplementary Information. Raw data are available from the corresponding author on reasonable request. Crystallographic data for the acylated derivatives of compounds 3d and 7e have been deposited with the Cambridge Crystallographic Data Centre, accession numbers CCDC 2197381 and 2197380, respectively.
References
Kurono, N. & Ohkuma, T. Catalytic asymmetric cyanation reactions. ACS Catal. 6, 989–1023 (2016).
Reetz, M. T., Kunisch, F. & Heitmann, P. Chiral Lewis acids for enantioselective C–C bond formation. Tetrahedron Lett. 27, 4721–4724 (1986).
Zuend, S. J., Coughlin, M. P., Lalonde, M. P. & Jacobsen, E. N. Scalable catalytic asymmetric Strecker syntheses of unnatural α-amino acids. Nature 461, 968–970 (2009).
Zhou, H. et al. Organocatalytic stereoselective cyanosilylation of small ketones. Nature 605, 84–89 (2022).
Gregory, R. J. Cyanohydrins in nature and the laboratory: biology, preparations, and synthetic applications. Chem. Rev. 99, 3649–3682 (1999).
Zeng, X.-P., Sun, J.-C., Liu, C.-, Ji, C.-B. & Peng, Y.-Y. Catalytic asymmetric cyanation reactions of aldehydes and ketones in total synthesis. Adv. Synth. Catal. 361, 3281–3305 (2019).
Rosenthaler, L. Durch enzyme bewirkte asymmetrische synthesen. Biochem. Z. 14, 238–253 (1908).
Kagan, H. B. in Comprehensive Asymmetric Catalysis, Vol. 1 (eds Jacobsen, E. N. et al.) 4–22 (Springer-Verlag, 1999).
Bredig, G. & Fiske, P. S. Beiträge zur chemischen physiologie und pathologie. Biochem. Z. 46, 7–23 (1912).
Sammis, G. M. & Jacobsen, E. N. Highly enantioselective, catalytic conjugate addition of cyanide to α,β-unsaturated imides. J. Am. Chem. Soc. 125, 4442–4443 (2003).
Sammis, G. M., Danjo, H. & Jacobsen, E. N. Cooperative dual catalysis: application to the highly enantioselective conjugate cyanation of unsaturated imides. J. Am. Chem. Soc. 126, 9928–9929 (2004).
Tanaka, Y., Kanai, M. & Shibasaki, M. A catalytic enantioselective conjugate addition of cyanide to enones. J. Am. Chem. Soc. 130, 6072–6073 (2008).
Tanaka, Y., Kanai, M. & Shibasaki, M. Catalytic enantioselective construction of β-quaternary carbons via a conjugate addition of cyanide to β,β-disubstituted α,β-unsaturated carbonyl compounds. J. Am. Chem. Soc. 132, 8862–8863 (2010).
Provencher, B. A., Bartelson, K. J., Liu, Y., Foxman, B. M. & Deng, L. Structural study-guided development of versatile phase-transfer catalysts for asymmetric conjugate additions of cyanide. Angew. Chem. Int. Ed. 50, 10565–10569 (2011).
Ito, Y., Kato, H., Imai, H. & Saegusa, T. A novel conjugate hydrocyanation with TiCl4-tert-butyl isocyanide. J. Am. Chem. Soc. 104, 6449–6450 (1982).
Jansen, B. J. M., Sengers, H. H. W. J. M., Bos, H. J. Y. & de Groot, A. A new stereoselective approach for the total synthesis of (±)-isotadeonal, (±)-polygodial, (±)-warburganal, and (±)-muzigadial. J. Org. Chem. 53, 855–859 (1988).
Nagata, W. & Yoshioka, M. in 25th Organic Reactions (eds Dauben, W. G. et al.) 255–476 (John Wiley & Sons, 1977).
Prelog, V. & Wilhelm, M. Untersuchungen über asymmetrische synthesen VI. Der reaktionsmechanismus und der sterische verlauf der asymmetrischen cyanhydrin-synthese. Helv. Chim. Acta 37, 1634–1660 (1954).
Hayashi, M., Miyamoto, Y., Inoue, T. & Oguni, N. Enantioselective trimethylsilylcyanation of some aldehydes catalyzed by chiral Schiff base-titanium alkoxide complexes. J. Org. Chem. 58, 1515–1522 (1993).
Hamashima, Y., Sawada, D., Kanai, M. & Shibasaki, M. A new bifunctional asymmetric catalysis: an efficient catalytic asymmetric cyanosilylation of aldehydes. J. Am. Chem. Soc. 121, 2641–2642 (1999).
Shenvi, R. A., O’Malley, D. P. & Baran, P. S. Chemoselectivity: the mother of invention in total synthesis. Acc. Chem. Res. 42, 530–541 (2009).
Lelais, G. & MacMillan, D. W. C. Modern strategies in organic catalysis: the advent and development of iminium activation. Aldrichimica Acta 39, 79–87 (2006).
Pirnot, M. T., Rankic, D. A., Martin, D. B. C. & MacMillan, D. W. C. Photoredox activation for the direct β-arylation of ketones and aldehydes. Science 339, 1593–1596 (2013).
Terrett, J. A., Clift, M. D. & MacMillan, D. W. C. Direct β-alkylation of aldehydes via photoredox organocatalysis. J. Am. Chem. Soc. 136, 6858–6861 (2014).
Seebach, D. Methods of reactivity umpolung. Angew. Chem. Int. Ed. 18, 239–258 (1979).
Barton, D. H. R., Jaszberenyl, J. C. & Theodorakis, E. A. The invention of radical reactions. Part XXIII new reactions: nitrile and thiocyanate transfer to carbon radicals from sulfonyl cyanides and sulfonyl isothiocyanates. Tetrahedron 48, 2613–2626 (1992).
Gaspar, B. & Carreira, E. M. Mild cobalt-catalyzed hydrocyanation of olefins with tosyl cyanide. Angew. Chem. Int. Ed. 46, 4519–4522 (2007).
Ren, R., Wu, Z., Xu, Y. & Zhu, C. C–C bond-forming strategy by manganese-catalyzed oxidative ring-opening cyanation and ethynylation of cyclobutanol derivatives. Angew. Chem. Int. Ed. 55, 2866–2869 (2016).
Shang, T.-Y. et al. Recent advances of 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4-CzIPN) in photocatalytic transformations. Chem. Commun. 55, 5408–5419 (2019).
Stradins, J. et al. Special features of the electrochemical oxidation of substituted 4-carboxy-1,4-dihydropyridines. Chem. Heterocycl. Compd. 36, 1177–1184 (2000).
Silvi, M., Verrier, C., Rey, Y. P., Buzzetti, L. & Melchiorre, P. Visible-light excitation of iminium ions enables the enantioselective catalytic β-alkylation of enals. Nat. Chem. 9, 868–873 (2017).
Yang, Y. W., Hechavarria Fonseca, M. T. H. & List, B. A metal-free transfer hydrogenation: organocatalytic conjugate reduction of α,β-unsaturated aldehydes. Angew. Chem. Int. Ed. 43, 6660–6662 (2004).
Suresh, R., Massad, I. & Marek, I. Stereoselective tandem iridium-catalyzed alkene isomerization-Cope rearrangement of ω-diene epoxides: efficient access to acyclic 1,6-dicarbonyl compounds. Chem. Sci. 12, 9328–9332 (2021).
Hopkinson, M., Richter, C., Schedler, M. & Glorius, F. An overview of N-heterocyclic carbenes. Nature 510, 485–496 (2014).
Chiang, P.-C., Kaeobamrung, J. & Bode, J. W. Enantioselective, cyclopentene-forming annulations via NHC-catalyzed benzoin−oxy-Cope reactions. J. Am. Chem. Soc. 129, 3520–3521 (2007).
Cardinal-David, B., Raup, D. E. A. & Scheidt, K. A. Cooperative N-heterocyclic carbene/Lewis acid catalysis for highly stereoselective annulation reactions with homoenolates. J. Am. Chem. Soc. 132, 5345–5347 (2010).
Huang, X. et al. Combining the catalytic enantioselective reaction of visible-light-generated radicals with a byproduct utilization system. Chem. Sci. 8, 7126–7313 (2017).
Acknowledgements
Financial support was provided by the Ministry for Science and Innovation AEI/10.13039/501100011033 (CEX2019-000925-S) and Agencia Estatal de Investigación (PID2019-106278GB-I00). M.B. thanks the Austrian Science Foundation (FWF, J4603-N) for an Erwin-Schrödinger postdoctoral fellowship. Y.B. thanks the Swiss National Science Foundation (P2BSP2_200098) for a postdoctoral fellowship. D.M. thanks the European Union for a Horizon 2020 Marie Skłodowska-Curie Fellowship (H2020-MSCA-IF-2019 894795). T.H.-F.W. thanks the Government of Catalonia for an FI Fellowship (2021FI−B00304). We thank P. Capurro for preliminary investigations, M. Martinez and J. Benet for X-ray crystallographic analysis and M. Giménez and C. Rivero for assistance with ozonolysis and hydrogenation experiments.
Author information
Authors and Affiliations
Contributions
M.B., Y.B. and T.H.-F.W. developed the reaction, investigated the substrate scope and studied the reaction mechanism. D.M. first observed the reactivity and performed the initial screening. All authors contributed to the experimental design and the interpretation of data. P.M. conceived and supervised the project. M.B., Y.B. and P.M. directed the project. M.B. and P.M. wrote the paper with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Table 1, Methods and References.
Supplementary Data 1
Crystallographic data of acylated derivative of compound 3d.
Supplementary Data 2
Crystallographic data of acylated derivative of compound 7e.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Berger, M., Ma, D., Baumgartner, Y. et al. Stereoselective conjugate cyanation of enals by combining photoredox and organocatalysis. Nat Catal 6, 332–338 (2023). https://doi.org/10.1038/s41929-023-00939-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-00939-y
This article is cited by
-
Reduction of unactivated alkyl chlorides enabled by light-induced single electron transfer
Science China Chemistry (2024)
-
NHC-catalyzed enantioselective access to β-cyano carboxylic esters via in situ substrate alternation and release
Nature Communications (2023)