Abstract
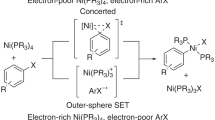
Polypyridine-ligated nickel complexes are widely used as privileged catalysts in a variety of cross-coupling reactions. The rapid adoption of these complexes is tentatively attributed to their ability to shuttle between different oxidation states and engage in electron-transfer reactions. However, these reactions are poorly understood in mechanistic terms. Here we investigate the reactivity of pseudohalide- and halide-ligated Ni(II) complexes, containing polypyridine ligands, in electron-transfer reactions. Specifically, Ni(II) halide complexes trigger comproportionation with Ni(0) with exceptional ease en route to Ni(I)Ln species, whereas the corresponding Ni(II) pseudohalide congeners are resistant to electron transfer, with Ni(I) pseudohalides being prone to disproportionation events. These observations are corroborated by electrochemical techniques and detailed quantum mechanical calculations. We also show that catalytically inactive Ni(II) pseudohalide complexes can be reactivated in the presence of exogeneous salts. From a broader perspective, this study provides rationalizations for overlooked and fundamental steps within the Ni-catalysed cross-coupling arena, thus offering blueprints for designing future Ni-catalysed reactions.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Experimental procedures and characterization data for the stochiometric experiments, catalysts and the synthesized compounds along with computational information are included in the Supplementary Information. Crystallographic data are available from the Cambridge Crystallographic Data Centre with the following codes: 3d (CCDC-2175355), 3c (CCDC-2175356), 3b (CCDC-2175354), 4a-Br (CCDC-2175353), 3a-Br (CCDC-2175357), [NiLi3Cl2(OtBu)3·2THF]2 (CCDC-2175358) and 3e (CCDC-2175352). Other data are available from the corresponding authors upon reasonable request.
References
Diccianni, J. B. & Diao, T. Mechanisms of nickel-catalyzed cross-coupling reactions. Trends Chem. 1, 830–844 (2019).
Diccianni, J., Lin, Q. & Diao, T. Mechanisms of nickel-catalyzed coupling reactions and applications in alkene functionalization. Acc. Chem. Res. 53, 906–919 (2020).
Tasker, S. Z., Standley, E. A. & Jamison, T. F. Recent advances in homogeneous nickel catalysis. Nature 509, 299–309 (2014).
Hazari, N., Melvin, P. R. & Beromi, M. M. Well-defined nickel and palladium precatalysts for cross-coupling. Nat. Rev. Chem. 1, 0025 (2017).
Everson, D. A. & Weix, D. J. Cross-electrophile coupling: principles of reactivity and selectivity. J. Org. Chem. 79, 4793–4798 (2014).
Mohadjer Beromi, M. et al. Mechanistic study of an improved Ni precatalyst for Suzuki–Miyaura reactions of aryl sulfamates: understanding the role of Ni(I) species. J. Am. Chem. Soc. 139, 922–936 (2017).
Barth, E. L. et al. Bis(dialkylphosphino)ferrocene-ligated nickel(II) precatalysts for Suzuki–Miyaura reactions of aryl carbonates. Organometallics 38, 3377–3387 (2019).
Mohadjer Beromi, M., Banerjee, G., Brudvig, G. W., Hazari, N. & Mercado, B. Q. Nickel(I) aryl species: synthesis, properties, and catalytic activity. ACS Catal. 8, 2526–2533 (2018).
Mohadjer Beromi, M. et al. Modifications to the aryl group of dppf-ligated Ni σ-aryl precatalysts: impact on speciation and catalytic activity in Suzuki–Miyaura coupling reactions. Organometallics 37, 3943–3955 (2018).
Yanagi, T., Somerville, R. J., Nogi, K., Martin, R. & Yorimitsu, H. Ni-catalyzed carboxylation of C(sp2)–S bonds with CO2: evidence for the multifaceted role of Zn. ACS Catal. 10, 2117–2123 (2020).
Day, C. S., Somerville, R. J. & Martin, R. Deciphering the dichotomy exerted by Zn(II) in the catalytic sp2 C–O bond functionalization of aryl esters at the molecular level. Nat. Catal. 4, 124–133 (2021).
Somerville, R. J., Hale, L. V. A., Gómez-Bengoa, E., Burés, J. & Martin, R. Intermediacy of Ni–Ni species in sp2 C–O bond cleavage of aryl esters: relevance in catalytic C–Si bond formation. J. Am. Chem. Soc. 140, 8771–8780 (2018).
Somerville, R. J. et al. Ni(I)–alkyl complexes bearing phenanthroline ligands: experimental evidence for CO2 insertion at Ni(I) centers. J. Am. Chem. Soc. 142, 10936–10941 (2020).
Heimbach, P. Changes in the coordination number of Ni(0) and Ni(I) compounds. Angew. Chem. Int. Ed. 3, 648 (1964).
Tsou, T. T. & Kochi, J. K. Mechanism of oxidative addition. Reaction of nickel(0) complexes with aromatic halides. J. Am. Chem. Soc. 101, 6319–6332 (1979).
Cundy, C. S. & Nöth, H. Metal-boron compounds: XI. Complexes derived from reactions of bis(triphenylphosphine)(π-ethylene)nickel with alkyl and boron halides. J. Organomet. Chem. 30, 135–143 (1971).
Porri, L., Gallazzi, M. C. & Vitulli, G. Complexes of nickel(I) with triphenylphosphine. Chem. Commun., 228–228 (1967).
Beattie, D. D., Lascoumettes, G., Kennepohl, P., Love, J. A. & Schafer, L. L. Disproportionation reactions of an organometallic Ni(I) amidate complex: scope and mechanistic Investigations. Organometallics 37, 1392–1399 (2018).
Dible, B. R., Sigman, M. S. & Arif, A. M. Oxygen-induced ligand dehydrogenation of a planar bis-μ-chloronickel(I) dimer featuring an NHC ligand. Inorg. Chem. 44, 3774–3776 (2005).
Schunn, R. A. Preparation and reactions of triethylphosphine complexes of zerovalent nickel, palladium, and platinum. Inorg. Chem. 15, 208–212 (1976).
Dürr, A. B., Fisher, H. C., Kalvet, I., Truong, K.-N. & Schoenebeck, F. Divergent reactivity of a dinuclear (NHC)nickel(I) catalyst versus nickel(0) enables chemoselective trifluoromethylselenolation. Angew. Chem. Int. Ed. 56, 13431–13435 (2017).
Kalvet, I., Guo, Q., Tizzard, G. J. & Schoenebeck, F. When weaker can be tougher: the role of oxidation state (I) in P- vs N-ligand-derived Ni-catalyzed trifluoromethylthiolation of aryl halides. ACS Catal. 7, 2126–2132 (2017).
Kapat, A., Sperger, T., Guven, S. & Schoenebeck, F. E-olefins through intramolecular radical relocation. Science 363, 391–396 (2019).
Yuan, M., Song, Z., Badir, S. O., Molander, G. A. & Gutierrez, O. On the nature of C(sp3)–C(sp2) bond formation in nickel-catalyzed tertiary radical cross-couplings: a case study of Ni/photoredox catalytic cross-coupling of alkyl radicals and aryl halides. J. Am. Chem. Soc. 142, 7225–7234 (2020).
Phapale, V. B., Guisán-Ceinos, M., Buñuel, E. & Cárdenas, D. J. Nickel-catalyzed cross-coupling of alkyl zinc halides for the formation of C(sp2)—C(sp3) bonds: scope and mechanism. Chem. Eur. J. 15, 12681–12688 (2009).
Lin, Q. & Diao, T. Mechanism of Ni-catalyzed reductive 1,2-dicarbofunctionalization of alkenes. J. Am. Chem. Soc. 141, 17937–17948 (2019).
Mohadjer Beromi, M., Brudvig, G. W., Hazari, N., Lant, H. M. C. & Mercado, B. Q. Synthesis and reactivity of paramagnetic nickel polypyridyl complexes relevant to C(sp2)–C(sp3)coupling reactions. Angew. Chem. Int. Ed. 58, 6094–6098 (2019).
Ting, S. I., Williams, W. L. & Doyle, A. G. Oxidative addition of aryl halides to a Ni(I)-bipyridine complex. J. Am. Chem. Soc. 144, 5575–5582 (2022).
Till, N. A., Oh, S., MacMillan, D. W. C. & Bird, M. J. The application of pulse radiolysis to the study of Ni(I) intermediates in Ni-catalyzed cross-coupling reactions. J. Am. Chem. Soc. 143, 9332–9337 (2021).
Ting, S. I. et al. 3d-d Excited states of Ni(II) complexes relevant to photoredox catalysis: spectroscopic identification and mechanistic implications. J. Am. Chem. Soc. 142, 5800–5810 (2020).
Huang, L., Ackerman, L. K. G., Kang, K., Parsons, A. M. & Weix, D. J. LiCl-accelerated multimetallic cross-coupling of aryl chlorides with aryl triflates. J. Am. Chem. Soc. 141, 10978–10983 (2019).
Powers, D. C., Anderson, B. L. & Nocera, D. G. Two-electron HCl to H2 photocycle promoted by Ni(II) polypyridyl halide complexes. J. Am. Chem. Soc. 135, 18876–18883 (2013).
Juliá-Hernández, F., Moragas, T., Cornella, J. & Martin, R. Remote carboxylation of halogenated aliphatic hydrocarbons with carbon dioxide. Nature 545, 84–88 (2017).
Li, Z. et al. Electrochemically enabled, nickel-catalyzed dehydroxylative cross-coupling of alcohols with aryl halides. J. Am. Chem. Soc. 143, 3536–3543 (2021).
Molander, G. A., Traister, K. M. & O’Neill, B. T. Engaging nonaromatic, heterocyclic tosylates in reductive cross-coupling with aryl and heteroaryl bromides. J. Org. Chem. 80, 2907–2911 (2015).
Dong, Z. & Macmillan, D. W. C. Metallaphotoredox-enabled deoxygenative arylation of alcohols. Nature 598, 451–456 (2021).
Zhang, Y., Xu, X. & Zhu, S. Nickel-catalysed selective migratory hydrothiolation of alkenes and alkynes with thiols. Nat. Commun. 10, 1752 (2019).
Smith, R. T. et al. Metallaphotoredox-catalyzed cross-electrophile Csp3–Csp3 coupling of aliphatic bromides. J. Am. Chem. Soc. 140, 17433–17438 (2018).
Peng, L., Li, Z. & Yin, G. Photochemical nickel-catalyzed reductive migratory cross-coupling of alkyl bromides with aryl bromides. Org. Lett. 20, 1880–1883 (2018).
van Gemmeren, M. et al. Switchable site-selective catalytic carboxylation of allylic alcohols with CO2. Angew. Chem. Int. Ed. 56, 6558–6562 (2017).
Tortajada, A., Börjesson, M. & Martin, R. Nickel-catalyzed reductive carboxylation and amidation reactions. Acc. Chem. Res. 54, 3941–3952 (2021).
Tortajada, A., Juliá‐Hernández, F., Börjesson, M., Moragas, T. & Martin, R. Transition‐metal‐catalyzed carboxylation reactions with carbon dioxide. Angew. Chem. Int. Ed. 57, 15948–15982 (2018).
Harris, C. M. & McKenzie, E. D. Nitrogenous chelate complexes of transition metals—III: bis-chelate complexes of nickel (II) with 1,10-phenanthroline, 2,2′-bipyridyl and analogous ligands. J. Inorg. Nucl. Chem. 29, 1047–1068 (1967).
Kinnunen, T.-J. J., Haukka, M., Pakkanen, T. T. & Pakkanen, T. A. Four-coordinated bipyridine complexes of nickel for ethene polymerization—the role of ligand structure. J. Organomet. Chem. 613, 257–262 (2000).
Fagnou, K. & Lautens, M. Halide effects in transition metal catalysis. Angew. Chem. Int. Ed. 41, 26–47 (2002).
Peng, L. et al. Ligand-controlled nickel-catalyzed reductive relay cross-coupling of alkyl bromides and aryl bromides. ACS Catal. 8, 310–313 (2018).
Tortajada, A., Ninokata, R. & Martin, R. Ni-catalyzed site-selective dicarboxylation of 1,3-dienes with CO2. J. Am. Chem. Soc. 140, 2050–2053 (2018).
Eremenko, I. L. et al. Bi- and mononuclear nickel(II) trimethylacetate complexes with pyridine bases as ligands. Inorg. Chem. 38, 3764–3773 (1999).
Acknowledgements
We thank ICIQ and FEDER/MCI−AEI/PGC2018-096839-B-I00 for financial support. C.S.D. thanks the European Union’s Horizon 2020 under the Marie Curie PREBIST grant agreement 754558. S.J.T. thanks Marie Sklodowska-Curie grant agreement No. 859910. O.G. thanks the NIGMS NIH (R35GM137797), the Camille and Henry Dreyfus Foundation and the Welch Foundation (A-2102-20220331) for funding and Texas A&M University HPRC resources (https://hprc.tamu.edu) for computational resources. We sincerely thank T. Skrydstrup for allowing revisions to be completed using his laboratory space and equipment, J. Benet for X-ray crystallographic data and G. Stoica for assistance with EPR experiments.
Author information
Authors and Affiliations
Contributions
C.S.D. conceived the project. C.S.D. designed and performed the experimental studies unless otherwise stated. S.J.T. performed parts of the electrochemical experiments, stochiometric reductions and catalytic reactions. A.R.-G. and A.R.G. performed the computational studies. O.G. supervised the computational research. R.M. supervised the experimental research. C.S.D and R.M. prepared the initial manuscript. All authors contributed to discussions, commented on and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Behrouz Notash and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–75 and Tables 1–4.
Supplementary Data 1
Crystallographic data for compound 3d.
Supplementary Data 2
Crystallographic data for compound 3c.
Supplementary Data 3
Crystallographic data for compound 3b.
Supplementary Data 4
Crystallographic data for compound 4a-Br.
Supplementary Data 5
Crystallographic data for compound 3a-Br.
Supplementary Data 6
Crystallographic data for compound [NiLi3Cl2(OtBu)3·2THF]2.
Supplementary Data 7
Crystallographic data for compound 3e.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Day, C.S., Rentería-Gómez, Á., Ton, S.J. et al. Elucidating electron-transfer events in polypyridine nickel complexes for reductive coupling reactions. Nat Catal 6, 244–253 (2023). https://doi.org/10.1038/s41929-023-00925-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-00925-4
This article is cited by
-
Electrophotocatalytic hydrogenation of imines and reductive functionalization of aryl halides
Nature Communications (2024)
-
Intramolecular hydroamidation of alkenes enabling asymmetric synthesis of β-lactams via transposed NiH catalysis
Nature Catalysis (2023)
-
One electron is better than two
Nature Catalysis (2023)