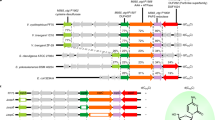
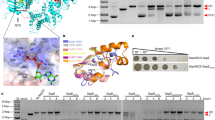
Abstract
Bacteria have evolved diverse mechanisms to cope with the constant threat of phage predation. DndABCDE-catalysed DNA phosphorothioate modification, in which the non-bridging oxygen of the DNA sugar-phosphate backbone is replaced by sulfur, can couple with proteins encoded by the dndFGH gene cluster to provide defensive protection, albeit via an obscure mode of action. Here we present structural and biochemical insights showing that the proteins DndF, DndG and DndH form a DndFGH complex and exhibit functions that are not detected in the individual proteins, including DNA nicking and translocation activities. Interestingly, DndFGH shows preferential affinity for phosphorothioate DNA, which suppresses the DNA-stimulated ATPase activity of the complex and consequently impedes its translocation and nicking activities. This phosphorothioate binding preference is believed to account for self/non-self discrimination and enable DndFGH to specifically introduce nicking damage in invasive DNA. This study expands our knowledge of the defence mechanisms involving epigenetic markers for the protection of self-DNA.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding authors upon reasonable request. The coordinates and structure factors of DndG and DndHCTD from E. coli B7A have been deposited in the Protein Data Bank under the accession numbers 7EXX and 7ES4, respectively. Source data are provided with this paper.
References
Tock, M. R. & Dryden, D. T. F. The biology of restriction and anti-restriction. Curr. Opin. Microbiol. 8, 466–472 (2005).
Yamaguchi, Y., Park, J. H. & Inouye, M. Toxin-antitoxin systems in bacteria and archaea. Annu. Rev. Genet. 45, 61–79 (2011).
Dy, R. L., Przybilski, R., Semeijn, K., Salmond, G. P. & Fineran, P. C. A widespread bacteriophage abortive infection system functions through a type IV toxin-antitoxin mechanism. Nucleic Acids Res. 42, 4590–4605 (2014).
Marraffini, L. A. CRISPR-Cas immunity in prokaryotes. Nature 526, 55–61 (2015).
Swarts, D. C. et al. DNA-guided DNA interference by a prokaryotic Argonaute. Nature 507, 258–261 (2014).
Thiaville, J. J. et al. Novel genomic island modifies DNA with 7-deazaguanine derivatives. Proc. Natl Acad. Sci. USA 113, E1452–E1459 (2016).
Chen, K., Zhao, B. S. & He, C. Nucleic acid modifications in regulation of gene expression. Cell Chem. Biol. 23, 74–85 (2016).
Wang, L. et al. Phosphorothioation of DNA in bacteria by dnd genes. Nat. Chem. Biol. 3, 709–710 (2007).
Wang, L. et al. DNA phosphorothioation is widespread and quantized in bacterial genomes. Proc. Natl Acad. Sci. USA 108, 2963–2968 (2011).
Zhou, X. et al. A novel DNA modification by sulphur. Mol. Microbiol. 57, 1428–1438 (2005).
Eckstein, F. Phosphorothioates, essential components of therapeutic oligonucleotides. Nucleic Acid Ther. 24, 374–387 (2014).
Xu, T., Yao, F., Zhou, X., Deng, Z. & You, D. A novel host-specific restriction system associated with DNA backbone S-modification in Salmonella. Nucleic Acids Res. 38, 7133–7141 (2010).
Cao, B. et al. Pathological phenotypes and in vivo DNA cleavage by unrestrained activity of a phosphorothioate-based restriction system in Salmonella. Mol. Microbiol. 93, 776–785 (2014).
Wang, L., Jiang, S., Deng, Z., Dedon, P. C. & Chen, S. DNA phosphorothioate modification—a new multi-functional epigenetic system in bacteria. FEMS Microbiol. Rev. 43, 109–122 (2019).
Cao, B. et al. Genomic mapping of phosphorothioates reveals partial modification of short consensus sequences. Nat. Commun. 5, 3951 (2014).
Chen, C. et al. Convergence of DNA methylation and phosphorothioation epigenetics in bacterial genomes. Proc. Natl Acad. Sci. USA 114, 4501–4506 (2017).
Tong, T. et al. Occurrence, evolution, and functions of DNA phosphorothioate epigenetics in bacteria. Proc. Natl Acad. Sci. USA 115, E2988–E2996 (2018).
Wu, X. et al. Epigenetic competition reveals density-dependent regulation and target site plasticity of phosphorothioate epigenetics in bacteria. Proc. Natl Acad. Sci. USA 117, 14322–14330 (2020).
Cao, B. et al. Nick-seq for single-nucleotide resolution genomic maps of DNA modifications and damage. Nucleic Acids Res. 48, 6715–6725 (2020).
Wei, Y. et al. Single-molecule optical mapping of the distribution of DNA phosphorothioate epigenetics. Nucleic Acids Res. 49, 3672–3680 (2021).
Wang, S. et al. SspABCD-SspFGH constitutes a new type of DNA phosphorothioate-based bacterial defense system. mBio 12, e00613–e00621 (2021).
Xiong, L. et al. A new type of DNA phosphorothioation-based antiviral system in archaea. Nat. Commun. 10, 1688 (2019).
Xiong, X. et al. SspABCD–SspE is a phosphorothioation-sensing bacterial defence system with broad anti-phage activities. Nat. Microbiol. 5, 917–928 (2020).
Liu, L. et al. Structural analysis of an l-cysteine desulfurase from an Ssp DNA phosphorothioation system. mBio 11, e00488 (2020).
Gan, R. et al. DNA phosphorothioate modifications influence the global transcriptional response and protect DNA from double-stranded breaks. Sci. Rep. 4, 6642 (2014).
Sharma, D. et al. Role of the conserved lysine within the Walker A motif of human DMC1. DNA Repair (Amst). 12, 53–62 (2013).
Kutnowski, N. et al. The 3-D structure of VNG0258H/RosR—A haloarchaeal DNA-binding protein in its ionic shell. J. Struct. Biol. 204, 191–198 (2018).
Steczkiewicz, K., Muszewska, A., Knizewski, L., Rychlewski, L. & Ginalski, K. Sequence, structure and functional diversity of PD-(D/E)XK phosphodiesterase superfamily. Nucleic Acids Res. 40, 7016–7045 (2012).
Pena, A. et al. The hexameric structure of a conjugative VirB4 protein ATPase provides new insights for a functional and phylogenetic relationship with DNA translocases. J. Biol. Chem. 287, 39925–39932 (2012).
Byrne, R. T., Schuller, J. M., Unverdorben, P., Forster, F. & Hopfner, K. P. Molecular architecture of the HerA–NurA DNA double-strand break resection complex. FEBS Lett. 588, 4637–4644 (2014).
Matilla, I. et al. The conjugative DNA translocase TrwB is a structure-specific DNA-binding protein. J. Biol. Chem. 285, 17537–17544 (2010).
Morris, P. D. & Raney, K. D. DNA helicases displace streptavidin from biotin-labeled oligonucleotides. Biochemistry 38, 5164–5171 (1999).
Zou, X. et al. Systematic strategies for developing phage resistant Escherichia coli strains. Nat. Commun. 13, 4491 (2022).
Liu, G. et al. Structural basis for the recognition of sulfur in phosphorothioated DNA. Nat. Commun. 9, 4689 (2018).
Chand, M. K. et al. Translocation-coupled DNA cleavage by the Type ISP restriction-modification enzymes. Nat. Chem. Biol. 11, 870–877 (2015).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Adams, P. D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D. Biol. Crystallogr. 58, 1948–1954 (2002).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. Biol. Crystallogr. 60, 2126–2132 (2004).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D. Biol. Crystallogr. 50, 760–763 (1994).
Winn, M. D., Murshudov, G. N. & Papiz, M. Z. Macromolecular TLS refinement in REFMAC at moderate resolutions. Methods Enzymol. 374, 300–321 (2003).
Moineau, S., Durmaz, E., Pandian, S. & Klaenhammer, T. R. Differentiation of two abortive mechanisms by using monoclonal antibodies directed toward lactococcal bacteriophage capsid proteins. Appl. Environ. Microbiol. 59, 208–212 (1993).
Acknowledgements
We thank the staff of the BL19U1 beamline of the National Facility for Protein Science in Shanghai (NFPS) at Shanghai Synchrotron Radiation Facility for assistance in data collection. This work was supported by grants from the National Natural Science Foundation of China (grant nos. 31925002 to Shi Chen, 32125001 to L.W., and 31872627 and 32170030 to G.W.), the National Key Research and Development Program of China (grant nos. 2022YFA0912200 to L.W., 2022YFA0912500 to Shi Chen. and 2020YFA0907300 to G.W.) and the Shanghai Jiao Tong University Scientific and Technological Innovation Fund (to G.W.).
Author information
Authors and Affiliations
Contributions
Shi Chen, G.W. and L.W. conceived the study and supervised the project. D.W., Y.T., Siwei Chen, Y.H. and X.C. performed the experiments. D.W., Y.T., Siwei Chen, Y.H., X.C., W.Z., Z.D., Z.L., L.W., G.W. and Shi Chen analysed the data. D.W., L.W., G.W. and Shi Chen wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Weixin Tang, Konstantin Severinov and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 SDS–PAGE gel and SEC-MALS analysis of the E.DndFGH complex.
a, Gel filtration profile of His-DndF, His-DndG, His-DndH and the DndFGH complex of B7A. The E.DndFGH complex was prepared by mixing His-DndF (64 μM), His-DndG (64 μM) and His-DndH (32 μM). b, A gel image of the SDS–PAGE analysis of eluted protein fractions from the size-exclusion chromatography. Results are representative of three independent experiments. c, No obvious DNA was observed in the eluted protein fractions by agarose gel electrophoresis. To exclude the involvement of DNA in the formation of the DndFGH complex, the mixture of DndF, DndG and DndH was treated with DNase I (0.033 U/µl) at 37 °C for 10 min. DNase I is capable of digesting DNA up to 5 μg under our experimental conditions. Results are representative of three independent experiments. d, SEC-MALS analysis shows that the E.DndFGH complex elutes with a molecular mass of 434.03 kDa, consistent with a theoretical value of 433.44 kDa when DndF, DndG and DndH, as shown in (a), assemble into a complex in a molar ratio of 2:2:1. The left and right axes represent the molecular weight and the light scattering detector reading, respectively. The black curve represents the calculated molecular weight. Results are representative of three independent experiments.
Extended Data Fig. 2 Nonspecific nicking activity of E.DndFGH.
Run-off sequencing of open circular pUC19 treated with DndFGH or Nt. BspQI. The peaks in the sequencing chromatogram dropped dramatically at the sequence-specific nicking site of Nt.BspQI. In contrast, no such sequencing peak drop was observed when DndFGH-nicked pUC19 DNA was used as a template despite the presence of the 5′-GAAC-3′/5′-GTTC-3′ motif (red underlined).
Extended Data Fig. 4 Translocation assays.
a–c, Streptavidin displacement over time from biotinylated DNA (250 ng) ranging from 150 to 750 bp is shown in the presence of E.DndFGH (a), E.DndFGHK1354A (b) or E.DndFGHD1574A (c). The molar ratio between E.DndFGH and DNA is approx. 5:1. d, The stacked column chart shows the relative percentages of free DNA and streptavidin-bound DNA (SA DNA). The results were obtained by quantitation of the gels in panel (a). Values represent the mean ± SD of three independent streptavidin displacement experiments. e, The steady-state rate of ATPase hydrolysis as a function of the DNA length and DNA concentration. Concentration is plotted as micromolar-base pairs (μMbp). Values represent the mean ± SD from three biologically independent experiments. f, g, The trends are fit with the Michaelis–Menten equation, allowing for the extraction of the Km (f) and Vmax (g) parameters. Data represent the mean ± SD from three biologically independent experiments.
Extended Data Fig. 5 A schematic model of PT modification-modulated DndFGH defence.
a, DndA/IscS and DndBCDE proteins act as the modification component to confer PT modification at a subset of 5′-GAAC-3′/5′-GTTC-3′ sites in host DNA, generating 5′-GPSAAC-3′/5′-GPSTTC-3′. b, As the restriction component, the DndFGH complex undergoes conformational changes to generate DNA nicking and translocation activities. Furthermore, PTs in self-DNA suppress the DNA-stimulated ATP hydrolysis activity of the DndFGH complex and consequently impedes its translocation and nicking activities, thus preventing autoimmunity.
Extended Data Fig. 6 Self-vs-nonself discrimination of DndFGH in vitro.
When PT-modified (PT+) pUC19 and unmodified (PT−) pACYC184 DNA were mixed at a 1:1 ratio and subjected to the nuclease digestion of E.DndFGH, PT+ pUC19 remained intact, whereas pACYC184 was specifically damaged. Without PT modification, PT− pUC19 DNA is also susceptible to E.DndFGH. Results are representative of three independent experiments.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2 and Figs. 1–3.
Supplementary Data
Supplementary Tables 3–5.
Source data
Source Data Fig. 1
Unprocessed plates, statistical source data.
Source Data Fig. 2
Unprocessed gels.
Source Data Fig. 3
Unprocessed gel, statistical source data.
Source Data Fig. 4
Unprocessed gels, statistical source data.
Source Data Fig. 5
Unprocessed gels, statistical source data.
Source Data Fig. 6
Unprocessed gel, statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Unprocessed gels, statistical source data.
Source Data Extended Data Fig. 1
Unprocessed gels, statistical source data.
Source Data Extended Data Fig. 4
Unprocessed gels, statistical source data.
Source Data Extended Data Fig. 6
Unprocessed gel.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, D., Tang, Y., Chen, S. et al. The functional coupling between restriction and DNA phosphorothioate modification systems underlying the DndFGH restriction complex. Nat Catal 5, 1131–1144 (2022). https://doi.org/10.1038/s41929-022-00884-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00884-2