Abstract
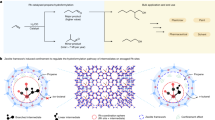
A high dispersion of the active metal phase of transition metals on oxide supports is important when designing efficient heterogeneous catalysts. Besides nanoparticles, clusters and even single metal atoms can be attractive for a wide range of reactions. However, many industrially relevant catalytic transformations suffer from structure sensitivity, where reducing the size of the metal particles below a certain size substantially lowers catalytic performance. A case in point is the low activity of small cobalt nanoparticles in the hydrogenation of CO and CO2. Here we show how engineering of catalytic sites at the metal–oxide interface in cerium oxide–zirconium dioxide (ceria–zirconia)-supported cobalt can overcome this structure sensitivity. Few-atom cobalt clusters dispersed on 3 nm cobalt(II)-oxide particles stabilized by ceria–zirconia yielded a highly active CO2 methanation catalyst with a specific activity higher than that of larger particles under the same conditions.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available from the corresponding author upon reasonable request. Coordinates of optimized structures used for DFT modelling are contained in Supplementary Data 1.
References
Yang, X. F. et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc. Chem. Res. 46, 1740–1748 (2013).
Liu, L. & Corma, A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 118, 4981–5079 (2018).
Somorjal, G. A. & Carrazza, J. Structure sensitivity of catalytic reactions. Ind. Eng. Chem. Fundam. 25, 63–69 (1986).
Van Santen, R. A. Complementary structure sensitive and insensitive catalytic relationships. Acc. Chem. Res. 42, 57–66 (2009).
Honkala, K. et al. Ammonia synthesis from first-principles calculations. Science 307, 555–558 (2005).
Dahl, S. et al. Role of steps in N2 activation on Ru(0001). Phys. Rev. Lett. 83, 1814–1817 (1999).
Bezemer, G. L. et al. Cobalt particle size effects in the Fischer–Tropsch reaction studied with carbon nanofiber supported catalysts. J. Am. Chem. Soc. 128, 3956–3964 (2006).
Vogt, C. et al. Unravelling structure sensitivity in CO2 hydrogenation over nickel. Nat. Catal. 1, 127–134 (2018).
van den Berg, R. et al. Structure sensitivity of Cu and CuZn catalysts relevant to industrial methanol synthesis. Nat. Commun. 7, 13057 (2016).
Crampton, A. S. et al. Structure sensitivity in the nonscalable regime explored via catalysed ethylene hydrogenation on supported platinum nanoclusters. Nat. Commun. 7, 10389 (2016).
Vogt, C., Kranenborg, J., Monai, M. & Weckhuysen, B. M. Structure sensitivity in steam and dry methane reforming over nickel: activity and carbon formation. ACS Catal. 10, 1428–1438 (2020).
Vogt, C., Monai, M., Kramer, G. J. & Weckhuysen, B. M. The renaissance of the Sabatier reaction and its applications on Earth and in space. Nat. Catal. 2, 188–197 (2019).
den Breejen, J. P. et al. On the origin of the cobalt particle size effects in Fischer−Tropsch catalysis. J. Am. Chem. Soc. 131, 7197–7203 (2009).
Parastaev, A. et al. Boosting CO2 hydrogenation via size-dependent metal–support interactions in cobalt/ceria-based catalysts. Nat. Catal. 3, 526–533 (2020).
Cargnello, M. et al. Control of metal nanocrystal size reveals metal–support interface role for ceria catalysts. Science 341, 771–773 (2013).
Ye, T. N. et al. Vacancy-enabled N2 activation for ammonia synthesis on an Ni-loaded catalyst. Nature 583, 391–395 (2020).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Fu, Q., Saltsburg, H. & Flytzani-Stephanopoulos, M. Active nonmetallic Au and Pt species on ceria-based water-gas shift catalysts. Science 301, 935–938 (2003).
Zhai, Y. et al. Alkali-stabilized Pt–OHx species catalyze low-temperature water-gas shift reactions. Science 329, 1633–1636 (2010).
Melaet, G. et al. Evidence of highly active cobalt oxide catalyst for the Fischer–Tropsch synthesis and CO2 hydrogenation. J. Am. Chem. Soc. 136, 2260–2263 (2014).
Zhao, K. et al. Unraveling and optimizing the metal–metal oxide synergistic effect in a highly active Cox(CoO)1−x catalyst for CO2 hydrogenation. J. Energy Chem 53, 241–250 (2020).
ten Have, I. C. et al. Uncovering the reaction mechanism behind CoO as active phase for CO2 hydrogenation. Nat. Commun. 13, 324 (2022).
Shin, H. K., Nam, I. S., Lee, J. S., Chung, J. S. & Moon, S. H. Catalytic properties of partially reduced cobalt wire in CO hydrogenation. Korean J. Chem. Eng. 13, 54–59 (1996).
Ra, E. C. et al. Recycling carbon dioxide through catalytic hydrogenation: recent key developments and perspectives. ACS Catal. 10, 11318–11345 (2020).
Tang, H. et al. Classical strong metal–support interactions between gold nanoparticles and titanium dioxide. Sci. Adv. 3, e1700231 (2017).
Matsubu, J. C., Yang, V. N. & Christopher, P. Isolated metal active site concentration and stability control catalytic CO2 reduction selectivity. J. Am. Chem. Soc. 137, 3076–3084 (2015).
Cao, S. et al. High-loading single Pt atom sites [Pt–O(OH)x] catalyze the CO PROX reaction with high activity and selectivity at mild conditions. Sci. Adv. 6, 3809–3826 (2020).
DeRita, L. et al. Catalyst architecture for stable single atom dispersion enables site-specific spectroscopic and reactivity measurements of CO adsorbed to Pt atoms, oxidized Pt clusters, and metallic Pt clusters on TiO2. J. Am. Chem. Soc. 139, 14150–14165 (2017).
Weststrate, C. J., van de Loosdrecht, J. & Niemantsverdriet, J. W. Spectroscopic insights into cobalt-catalyzed Fischer–Tropsch synthesis: a review of the carbon monoxide interaction with single crystalline surfaces of cobalt. J. Catal. 342, 1–16 (2016).
Wang, F. et al. Active site dependent reaction mechanism over Ru/CeO2 catalyst toward CO2 methanation. J. Am. Chem. Soc. 138, 6298–6305 (2016).
Aldana, P. A. U. et al. Catalytic CO2 valorization into CH4 on Ni-based ceria–zirconia. Reaction mechanism by operando IR spectroscopy. Catal. Today 215, 201–207 (2013).
Lin, S. S.-Y., Daimon, H. & Ha, S. Y. Co/CeO2–ZrO2 catalysts prepared by impregnation and coprecipitation for ethanol steam reforming. Appl. Catal. A 366, 252–261 (2009).
Vayssilov, G. N., Mihaylov, M., Petkov, P. S., Hadjiivanov, K. I. & Neyman, K. M. Reassignment of the vibrational spectra of carbonates, formates, and related surface species on ceria: a combined density functional and infrared spectroscopy investigation. J. Phys. Chem. C 115, 23435–23454 (2011).
Daturi, M. et al. Study of bulk and surface reduction by hydrogen of CexZr1–xO2 mixed oxides followed by FTIR spectroscopy and magnetic balance. J. Phys. Chem. B 103, 4884–4891 (1999).
Wang, X., Shi, H. & Szanyi, J. Controlling selectivities in CO2 reduction through mechanistic understanding. Nat. Commun. 8, 513 (2017).
Tuxen, A. et al. Size-dependent dissociation of carbon monoxide on cobalt nanoparticles. J. Am. Chem. Soc. 135, 2273–2278 (2013).
Bowers, C. R. & Weitekamp, D. P. Parahydrogen and synthesis allow dramatically enhanced nuclear alignment. J. Am. Chem. Soc. 109, 5541–5542 (1987).
Corma, A., Salnikov, O. G., Barskiy, D. A., Kovtunov, K. V. & Koptyug, I. V. Single-atom gold catalysis in the context of developments in parahydrogen-induced polarization. Chemistry 21, 7012–7015 (2015).
Pokochueva, E. V., Burueva, D. B., Salnikov, O. G. & Koptyug, I. V. Heterogeneous catalysis and parahydrogen-induced polarization. ChemPhysChem 22, 1421–1440 (2021).
Kovtunov, K. V. et al. Catalytic hydrogenation with parahydrogen: a bridge from homogeneous to heterogeneous catalysis. Pure Appl. Chem. 92, 1029–1046 (2020).
Galhardo, T. S. et al. Optimizing active sites for high CO selectivity during CO2 hydrogenation over supported nickel catalysts. J. Am. Chem. Soc. 143, 4268–4280 (2021).
Yuan, Q. et al. Ordered mesoporous Ce1−xZrxO2 solid solutions with crystalline walls. J. Am. Chem. Soc. 129, 6698–6699 (2007).
Altantzis, T. et al. Three-dimensional quantification of the facet evolution of Pt nanoparticles in a variable gaseous environment. Nano Lett. 19, 477–481 (2018).
Artiglia, L. et al. Introducing time resolution to detect Ce3+ catalytically active sites at the Pt/CeO2 interface through ambient pressure X-ray photoelectron spectroscopy. J. Phys. Chem. Lett. 8, 102–108 (2017).
Skála, T., Šutara, F., Prince, K. C. & Matolín, V. Cerium oxide stoichiometry alteration via Sn deposition: influence of temperature. J. Electron Spectros. Relat. Phenomena 169, 20–25 (2009).
Stadnichenko, A. I. et al. Study of active surface centers of Pt/CeO2 catalysts prepared using radio-frequency plasma sputtering technique. Surf. Sci. 679, 273–283 (2019).
Kosinov, N. et al. Confined carbon mediating dehydroaromatization of methane over Mo/ZSM-5. Angew. Chem. Int. Ed. 57, 1016–1020 (2018).
Pravica, M. G. & Weitekamp, D. P. Net NMR alignment by adiabatic transport of parahydrogen addition products to high magnetic field. Chem. Phys. Lett. 145, 255–258 (1988).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Park, K. W. & Kolpak, A. M. Understanding photocatalytic overall water splitting on CoO nanoparticles: effects of facets, surface stoichiometry, and the CoO/water interface. J. Catal. 365, 115–124 (2018).
Acknowledgements
This research was supported by the Applied and Engineering Sciences division of the Netherlands Organization for Scientific Research through the Alliander (now Qirion) Perspective programme on Plasma Conversion of CO2. We acknowledge Diamond Light Source for time on beamline B18 under proposal no. SP20715-1. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 823717 – ESTEEM3. S.B. acknowledges support from the European Research Council (ERC Consolidator Grant no. 815128 REALNANO) and T.A. acknowledges funding from the University of Antwerp Research fund (BOF). A.B. received funding from the European Union under grant agreement no. 823717 – ESTEEM3. The authors acknowledge funding through the Hercules grant (FWO, University of Antwerp) no. I003218N, ‘Infrastructure for imaging nanoscale processes in gas/vapour or liquid environments’. I.V.K., D.B.B. and E.V.P. acknowledge the Russian Ministry of Science and Higher Education (contract no. 075-15-2021-580) for financial support with parahydrogen-based studies. Experiments using synchrotron radiation XPS were performed at the CIRCE beamline at ALBA Synchrotron with the collaboration of ALBA staff. F. Oropeza Palacio and R. C. J. van de Poll are acknowledged for help with RPES measurements.
Author information
Authors and Affiliations
Contributions
A.P. synthesized and characterized the set of ceria–zirconia samples (TPR, XRD and CO chemisorption and IR spectroscopy). A.P. and E.H.O. performed catalytic measurements. V.M., A.P. and N.K. performed in situ NAP-XPS and operando XAS measurements and interpreted the results. A.J.F.H. performed TEM measurements with an in situ holder. T.F.K. performed quasi-in situ XPS. J.F.M.S. and J.J.C.S. wrote the MATLAB script for rapid-scan FTIR measurements. A.P. and E.U. performed H2–D2 exchange experiments. I.V.K., D.B.B. and E.V.P. performed and interpreted experiments with parahydrogen. T.A., P.L., A.B., S.B., A.P. and N.K. performed and interpreted in situ STEM–EELS experiments. A.P., V.M., N.K. and E.J.M.H. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Jochen Lauterbach, Clifford Bowers and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–88, Notes 1–12, Tables 1–11, equation 1 and references.
Supplementary Data
Coordinates of optimized structures used for DFT modelling.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parastaev, A., Muravev, V., Osta, E.H. et al. Breaking structure sensitivity in CO2 hydrogenation by tuning metal–oxide interfaces in supported cobalt nanoparticles. Nat Catal 5, 1051–1060 (2022). https://doi.org/10.1038/s41929-022-00874-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00874-4
This article is cited by
-
Facilitating the dry reforming of methane with interfacial synergistic catalysis in an Ir@CeO2−x catalyst
Nature Communications (2024)
-
Interfacial catalysis of metal-oxide nanocatalysts in CO2 hydrogenation to value-added C1 chemicals
Surface Science and Technology (2023)