Abstract
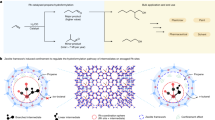
The oxidative dehydrogenation of propane using CO2 (CO2-ODP) is a promising technique for high-yield propylene production and CO2 utilization. Unfortunately the efficiency of existing catalysts is limited, and therefore, developing a highly efficient catalyst for CO2-ODP is of great importance for the chemical industry. Here we report a Pt–Co–In ternary nanoalloy on CeO2 that has a (Pt1−xCox)2In3 pseudo-binary alloy structure and exhibits very high catalytic activity, C3H6 selectivity, stability and CO2 utilization efficiency at 550 °C. Alloying platinum with indium and cobalt significantly improves the C3H6 selectivity and CO2 reduction ability, respectively. The cobalt species provide a high density of states near the Fermi level, which lowers the energy barrier of CO2 reduction. The stability of the catalyst is greatly enhanced by combining the strong CO2 activation ability of the alloy with the oxygen releasing ability of the CeO2 support, which facilitates Mars–van Krevelen-type coke combustion.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Atomic coordinates of the optimized computational models are provided as Supplementary Data 1 with this paper. Other data that support the findings of this study can be found in the article and the Supplementary Information; this information is also available from the corresponding author upon request.
References
Amghizar, I., Vandewalle, L. A., Van Geem, K. M. & Marin, G. B. New trends in olefin production. Engineering 3, 171–178 (2017).
Sattler, J. J. H. B., Ruiz-Martinez, J., Santillan-Jimenez, E. & Weckhuysen, B. M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 114, 10613–10653 (2014).
Bhasin, M. M., McCain, J. H., Vora, B. V., Imai, T. & Pujadó, P. R. Dehydrogenation and oxydehydrogenation of paraffins to olefins. Appl. Catal. A 221, 397–419 (2001).
Wang, S. & Zhu, Z. H. Catalytic conversion of alkanes to olefins by carbon dioxide oxidative dehydrogenation—a review. Energy Fuels 18, 1126–1139 (2004).
Atanga, M. A., Rezaei, F., Jawad, A., Fitch, M. & Rownaghi, A. A. Oxidative dehydrogenation of propane to propylene with carbon dioxide. Appl. Catal. B 220, 429–445 (2018).
Ansari, M. B. & Park, S. E. Carbon dioxide utilization as a soft oxidant and promoter in catalysis. Energy Environ. Sci. 5, 9419–9437 (2012).
Gomez, E. et al. Combining CO2 reduction with propane oxidative dehydrogenation over bimetallic catalysts. Nat. Commun. 9, 1398 (2018).
Ruthwik, N., Kavya, D., Shadab, A., Lingaiah, N. & Sumana, C. Thermodynamic analysis of chemical looping combustion integrated oxidative dehydrogenation of propane to propylene with CO2. Chem. Eng. Process. 153, 107959 (2020).
Farnaz, T. Z., Abbas, T., Khodayer, G. & Saeed, S. Thermodynamic equilibrium analysis of propane dehydrogenation with carbon dioxide and side reactions. Chem. Eng. Commun. 203, 557–565 (2016).
Osaki, T. & Mori, T. Kinetics of the reverse-Boudouard reaction over supported nickel catalysts. React. Kinet. Catal. Lett. 89, 333–339 (2006).
Jacobson, M. Z. Review of solutions to global warming, air pollution, and energy security. Energy Environ. Sci. 2, 148–173 (2009).
Tollefson, J. World’s carbon emissions set to spike by 2% in 2017. Nature 551, 283–283 (2017).
Shishido, T., Shimamura, K., Teramura, K. & Tanaka, T. Role of CO2 in dehydrogenation of propane over Cr-based catalysts. Catal. Today 185, 151–156 (2012).
Botavina, M. A. et al. Towards efficient catalysts for the oxidative dehydrogenation of propane in the presence of CO2: Cr/SiO2 systems prepared by direct hydrothermal synthesis. Catal. Sci. Technol. 6, 840–850 (2016).
Xu, B., Zheng, B., Hua, W., Yue, Y. & Gao, Z. Support effect in dehydrogenation of propane in the presence of CO2 over supported gallium oxide catalysts. J. Catal. 239, 470–477 (2006).
Chen, M. et al. Study in support effect of In2O3/MOx (M = Al, Si, Zr) catalysts for dehydrogenation of propane in the presence of CO2. Appl. Catal. A 407, 20–28 (2011).
Nowicka, E. et al. Elucidating the role of CO2 in the soft oxidative dehydrogenation of propane over ceria-based catalysts. ACS Catal. 8, 3454–3468 (2018).
Baek, J., Yun, H. J., Yun, D., Choi, Y. & Yi, J. Preparation of highly dispersed chromium oxide catalysts supported on mesoporous silica for the oxidative dehydrogenation of propane using CO2: insight into the nature of catalytically active chromium sites. ACS Catal. 2, 1893–1903 (2012).
Wang, H. M., Chen, Y., Yan, X., Lang, W. Z. & Guo, Y. J. Cr doped mesoporous silica spheres for propane dehydrogenation in the presence of CO2: effect of Cr adding time in sol–gel process. Microporous Mesoporous Mater. 284, 69–77 (2019).
Jeon, J., Kon, K., Toyao, T., Shimizu, K. & Furukawa, S. Design of Pd-based pseudo-binary alloy catalysts for highly active and selective NO reduction. Chem. Sci. 10, 4148–4162 (2019).
Nakaya, Y., Miyazaki, M., Yamazoe, S., Shimizu, K. & Furukawa, S. Active, Selective, and durable catalyst for alkane dehydrogenation based on a well-designed trimetallic alloy. ACS Catal. 10, 5163–5172 (2020).
Nakaya, Y., Hirayama, J., Yamazoe, S., Shimizu, K. & Furukawa, S. Single-atom Pt in intermetallics as an ultrastable and selective catalyst for propane dehydrogenation. Nat. Commun. 11, 2838 (2020).
Sun, G. et al. Breaking the scaling relationship via thermally stable Pt/Cu single atom alloys for catalytic dehydrogenation. Nat. Commun. 9, 4454 (2018).
Ryoo, R. et al. Rare-earth–platinum alloy nanoparticles in mesoporous zeolite for catalysis. Nature 585, 221–224 (2020).
Liu, B. & Greeley, J. Decomposition pathways of glycerol via C–H, O–H, and C–C bond scission on Pt(111): a density functional theory study. J. Phys. Chem. C 115, 19702–19709 (2011).
Álvarez, A. et al. CO2 activation over catalytic surfaces. ChemPhysChem 18, 3135–3141 (2017).
Studt, F. et al. Discovery of a Ni–Ga catalyst for carbon dioxide reduction to methanol. Nat. Chem. 6, 320–324 (2014).
Furukawa, S. & Komatsu, T. Intermetallic compounds: promising inorganic materials for well-structured and electronically modified reaction environments for efficient catalysis. ACS Catal. 7, 735–765 (2017).
Pakhare, D. & Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 43, 7813–7837 (2014).
Kopač, D., Likozar, B. & Huš, M. How size matters: electronic, cooperative, and geometric effect in perovskite-supported copper catalysts for CO2 reduction. ACS Catal. 10, 4092–4102 (2020).
Zhang, W. et al. Size dependence of Pt catalysts for propane dehydrogenation: from atomically dispersed to nanoparticles. ACS Catal. 10, 12932–12942 (2020).
Zhu, J. et al. Size-dependent reaction mechanism and kinetics for propane dehydrogenation over Pt catalysts. ACS Catal. 5, 6310–6319 (2015).
Lin, C. F., Mohney, S. E. & Chang, Y. A. Phase equilibria in the Pt–In–P system. J. Appl. Phys. 74, 4398–4402 (1993).
Barbosa, R. L. et al. Methanol steam reforming over indium-promoted Pt/Al2O3 catalyst: nature of the active surface. J. Phys. Chem. C 117, 6143–6150 (2013).
Nguyen, T. D., Zheng, W., Celik, F. E. & Tsilomelekis, G. T. CO2-assisted ethane oxidative dehydrogenation over MoOx catalysts supported on reducible CeO2–TiO2. Catal. Sci. Technol. 11, 5791–5801 (2021).
Melaet, G. et al. Evidence of highly active cobalt oxide catalyst for the Fischer–Tropsch synthesis and CO2 hydrogenation. J. Am. Chem. Soc. 136, 2260–2263 (2014).
Wang, L., Liu, H., Chen, Y. & Yang, S. Reverse water–gas shift reaction over co-precipitated Co–CeO2 catalysts: effect of Co content on selectivity and carbon formation. Int. J. Hydrog. Energy 42, 3682–3689 (2017).
Wang, Y., Furukawa, S. & Yan, N. Identification of an active NiCu catalyst for nitrile synthesis from alcohol. ACS Catal. 9, 6681–6691 (2019).
Hammer, B. & Norskov, J. K. Why gold is the noblest of all the metals. Nature 376, 238–240 (1995).
LiBretto, N. J., Yang, C., Ren, Y., Zhang, G. & Miller, J. T. Identification of surface structures in Pt3Cr intermetallic nanocatalysts. Chem. Mater. 31, 1597–1609 (2019).
Ankudinov, A. & Ravel, B. Real-space multiple-scattering calculation and interpretation of X-ray-absorption near-edge structure. Phys. Rev. B 58, 7565–7576 (1998).
Segall, M. D. et al. First-principles simulation: ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 14, 2717–2744 (2002).
Hammer, B., Hansen, L. B. & Nørskov, J. K. Improved adsorption energetics within density-functional theory using revised Perdew–Burke–Ernzerhof functionals. Phys. Rev. B 59, 7413–7421 (1999).
Tkatchenko, A. & Scheffler, M. Accurate molecular van der Waals interactions from ground-state electron density and free-atom reference data. Phys. Rev. Lett. 102, 6–9 (2009).
Hu, K. et al. Boosting electrochemical water splitting: via ternary NiMoCo hybrid nanowire arrays. J. Mater. Chem. A 7, 2156–2164 (2019).
Jana, R. & Peter, S. C. One-pot solvothermal synthesis of ordered intermetallic Pt2In3 as stable and efficient electrocatalyst towards direct alcohol fuel cell application. J. Solid State Chem. 242, 133–139 (2016).
Ye, J., Liu, C. & Ge, Q. A DFT study of methanol dehydrogenation on the PdIn(110) surface. Phys. Chem. Chem. Phys. 14, 16660–16667 (2012).
Fischer, T. H. & Almlöf, J. General methods for geometry and wave function optimization. J. Phys. Chem. 96, 9768–9774 (1992).
Govind, N., Petersen, M., Fitzgerald, G., King-Smith, D. & Andzelm, J. A generalized synchronous transit method for transition state location. Comput. Mater. Sci. 28, 250–258 (2003).
Halgren, T. A. & Lipscomb, W. N. The synchronous-transit method for determining reaction pathways and locating molecular transition states. Chem. Phys. Lett. 49, 225–232 (1977).
Wang, J. et al. A highly selective and stable ZnO–ZrO2 solid solution catalyst for CO2 hydrogenation to methanol. Sci. Adv. 3, 1–11 (2017).
Schoonheydt, R. A. & Weckhuysen, B. M. Physisorption and chemisorption of alkanes and alkenes in H-FAU: a combined ab initio–statistical thermodynamics study. Phys. Chem. Chem. Phys. 11, 2794–2798 (2009).
Acknowledgements
This work was supported by JSPS KAKENHI (grant numbers 17H01341 to K.S., 17H04965 to S.F. and 20H02517 to S.F.), MEXT project Element Strategy Initiative (JPMXP0112101003 to K.S.), JST CREST (JPMJCR17J3 to K.S.), JST PRESTO (JPMJPR19T7 to S.F.) and the Collaborative Research Projects of Laboratory for Materials and Structures, Institute of Innovative Research, Tokyo Institute of Technology to S.F. The XAFS analysis was performed with the approval of JASRI (number 2019B1620 and 2019B1469 to S.F.). We thank the technical staff of the Research Institute for Electronic Science, Y. Nakasaka and K.W. Ting, Hokkaido University for help with HAADF-STEM, Raman and XPS analyses, respectively. Computation time was provided by the supercomputer systems in the Institute for Chemical Research, Kyoto University.
Author information
Authors and Affiliations
Contributions
S.F. and F.X. designed the research and co-wrote the manuscript in discussion. F.X. performed all the experimetal work. Y.N. contributed to the XAFS and XPS studies. S.F. conducted all the computational and kinetic studies. S.Y. contributed to the free-energy calculation. S.F., F.X., Y.N., S.Y. and K.S. discussed the data and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Feng-Shou Xiao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–39, Tables 1–9 and Note 1.
Supplementary Data 1
Atomic coordinates of optimized computational models.
Rights and permissions
About this article
Cite this article
Xing, F., Nakaya, Y., Yasumura, S. et al. Ternary platinum–cobalt–indium nanoalloy on ceria as a highly efficient catalyst for the oxidative dehydrogenation of propane using CO2. Nat Catal 5, 55–65 (2022). https://doi.org/10.1038/s41929-021-00730-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-021-00730-x
This article is cited by
-
Distance effect of single atoms on stability of cobalt oxide catalysts for acidic oxygen evolution
Nature Communications (2024)
-
Low-temperature non-equilibrium synthesis of anisotropic multimetallic nanosurface alloys for electrochemical CO2 reduction
Nature Synthesis (2023)
-
Advanced Kinetic and Titration Strategies for Assessing the Intrinsic Kinetics on Oxide and Sulfide Catalysts
Topics in Catalysis (2023)
-
Promotional nature of Sn on Pt/CeO2 for the oxidative dehydrogenation of propane with carbon dioxide
Nano Research (2023)
-
Atomic dispersion of bulk/nano metals to atomic-sites catalysts and their application in thermal catalysis
Nano Research (2023)