Abstract
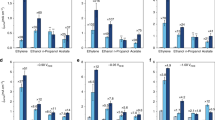
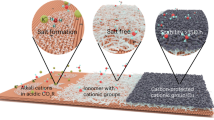
Artificial carbon fixation contributes to closing the anthropogenic carbon cycle. However, large-scale conversion of CO2 into selective products remains a challenge. Coupled thermal–electrochemical catalysis could offer an attractive approach to upgrading CO2 into value-added products if selective electrocatalysts and integrated devices were developed. Here we identify a mechanistic route to selectively producing either CO or CH4 with high selectivity (>95%) using Ir–ceria-based catalysts in an intermediate-temperature (400 °C) CO2 electrolyser that operates at low overpotential and ambient pressure. We show that tuning of the Ir–O hybridization by controlling the Ir speciation can alter the catalyst surface chemical environment, enabling the stabilization of specific transition states for the production of either CO or CH4 during electrocatalysis. By achieving CO2 electrohydrogenation in tandem with light-alkane electrodehydrogenation, we further demonstrate that such an advanced electrolyser could be extended to the upgrade of different carbon resources in one-step, significantly enhancing the techno-economic feasibilty of the process.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data are provided with this paper. Any additional data are available upon request from the corresponding author. Additional methods and results are provided in the Supplementary Information.
Change history
30 April 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41929-021-00623-z
References
Mahaffy, P. G., Matlin, S. A., Holme, T. A. & MacKellar, J. Systems thinking for education about the molecular basis of sustainability. Nat. Sustain. 2, 362–370 (2019).
Jacobson, T. A. et al. Direct human health risks of increased atmospheric carbon dioxide. Nat. Sustain. 2, 691–701 (2019).
Jiang, M. et al. The fate of carbon in a mature forest under carbon dioxide enrichment. Nature 580, 227–231 (2020).
Zimmerman, J. B., Anastas, P. T., Erythropel, H. C. & Leitner, W. Designing for a green chemistry future. Science 367, 397–400 (2020).
Gomez, E., Yan, B., Kattel, S. & Chen, J. G. Carbon dioxide reduction in tandem with light-alkane dehydrogenation. Nat. Rev. Chem. 3, 638–649 (2019).
Ye, R.-P. et al. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 10, 5698 (2019).
Field, C. B. & Mach, K. J. Rightsizing carbon dioxide removal. Science 356, 706–707 (2017).
Patel, H. A., Byun, J. & Yavuz, C. T. Carbon dioxide capture adsorbents: chemistry and methods. ChemSusChem 10, 1303–1317 (2017).
Roy, S., Cherevotan, A. & Peter, S. C. Thermochemical CO2 hydrogenation to single carbon products: scientific and technological challenges. ACS Energy Lett. 3, 1938–1966 (2018).
Stamenkovic, V. R., Strmcnik, D., Lopes, P. P. & Markovic, N. M. Energy and fuels from electrochemical interfaces. Nat. Mater. 16, 57–69 (2017).
Wang, W.-H., Himeda, Y., Muckerman, J. T., Manbeck, G. F. & Fujita, E. CO2 hydrogenation to formate and methanol as an alternative to photo- and electrochemical CO2 reduction. Chem. Rev. 115, 12936–12973 (2015).
Tackett, B. M., Sheng, W. & Chen, J. G. Opportunities and challenges in utilizing metal-modified transition metal carbides as low-cost electrocatalysts. Joule 1, 253–263 (2017).
Fan, L. et al. Strategies in catalysts and electrolyzer design for electrochemical CO2 reduction toward C2+ products. Sci. Adv. 6, eaay3111 (2020).
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Meng, Y. et al. Highly active oxygen evolution integrated with efficient CO2 to CO electroreduction. Proc. Natl Acad. Sci. USA 116, 23915–23922 (2019).
Zheng, T., Jiang, K. & Wang, H. Recent advances in electrochemical CO2-to-CO conversion on heterogeneous catalysts. Adv. Mater. 30, 1802066 (2018).
Ma, M. et al. Insights into the carbon balance for CO2 electroreduction on Cu using gas diffusion electrode reactor designs. Energy Environ. Sci. 13, 977–985 (2020).
Ren, S. et al. Molecular electrocatalysts can mediate fast, selective CO2 reduction in a flow cell. Science 365, 367–369 (2019).
Dinh, C.-T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Li, C. W., Ciston, J. & Kanan, M. W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 508, 504–507 (2014).
Chen, Y., Li, C. W. & Kanan, M. W. Aqueous CO2 reduction at very low overpotential on oxide-derived Au nanoparticles. J. Am. Chem. Soc. 134, 19969–19972 (2012).
Ding, D. et al. A novel low-thermal-budget approach for the co-production of ethylene and hydrogen via the electrochemical non-oxidative deprotonation of ethane. Energy Environ. Sci. 11, 1710–1716 (2018).
Zhang, X., Ye, L., Li, H., Chen, F. & Xie, K. Electrochemical dehydrogenation of ethane to ethylene in a solid oxide electrolyzer. ACS Catal. 10, 3505–3513 (2020).
Duan, C. et al. Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 4, 230–240 (2019).
Van Deelen, T. W., Hernández Mejía, C. & de Jong, K. P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2, 955–970 (2019).
Yang, X. et al. Low pressure CO2 hydrogenation to methanol over gold nanoparticles activated on a CeOx/TiO2 interface. J. Am. Chem. Soc. 137, 10104–10107 (2015).
Hua, B. et al. A coupling for success: controlled growth of Co/CoOx nanoshoots on perovskite mesoporous nanofibres as high-performance trifunctional electrocatalysts in alkaline condition. Nano Energy 32, 247–254 (2017).
Lam, E. et al. CO2 hydrogenation on Cu/Al2O3: role of the metal/support interface in driving activity and selectivity of a bifunctional catalyst. Angew. Chem. Int. Ed. 58, 13989–13996 (2019).
Li, M., Hua, B., Chen, J., Zhong, Y. & Luo, J.-L. Charge transfer dynamics in RuO2/perovskite nanohybrid for enhanced electrocatalysis in solid oxide electrolyzers. Nano Energy 57, 186–194 (2019).
Zhang, L., Zhou, M., Wang, A. & Zhang, T. Selective hydrogenation over supported metal catalysts: from nanoparticles to single atoms. Chem. Rev. 120, 683–733 (2020).
Jeong, H. et al. Highly durable metal ensemble catalysts with full dispersion for automotive applications beyond single-atom catalysts. Nat. Catal. 3, 368–375 (2020).
Beniya, A. & Higashi, S. Towards dense single-atom catalysts for future automotive applications. Nat. Catal. 2, 590–602 (2019).
Lou, Y. et al. Pocketlike active site of Rh1/MoS2 single-atom catalyst for selective crotonaldehyde hydrogenation. J. Am. Chem. Soc. 141, 19289–19295 (2019).
Krewald, V., Neese, F. & Pantazis, D. A. Redox potential tuning by redox-inactive cations in nature’s water oxidizing catalyst and synthetic analogues. Phys. Chem. Chem. Phys. 18, 10739–10750 (2016).
Hong, W. T. et al. Probing LaMO3 metal and oxygen partial density of states using X-ray emission, absorption, and photoelectron spectroscopy. J. Phys. Chem. C 119, 2063–2072 (2015).
Winterbottom, W. L. Equilibrium shape of a small particle in contact with a foreign substrate. Acta Metall. 15, 303–310 (1967).
Wulff, G. X. X. V. Zur frage der geschwindigkeit des wachsthums und der auflösung der krystallflächen. Z. Kristallogr. Cryst. Mater. 34, 449 (1901).
Molina, L. M. & Hammer, B. Active role of oxide support during CO oxidation at Au/MgO. Phys. Rev. Lett. 90, 206102 (2003).
Du, J., Sun, X., Chen, J. & Jiang, G. A theoretical study on small iridium clusters: structural evolution, electronic and magnetic properties, and reactivity predictors. J. Phys. Chem. A 114, 12825–12833 (2010).
Henkelman, G., Arnaldsson, A. & Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 36, 354–360 (2006).
Sanville, E., Kenny, S. D., Smith, R. & Henkelman, G. Improved grid‐based algorithm for Bader charge allocation. J. Comput. Chem. 28, 899–908 (2007).
Tang, W., Sanville, E. & Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 21, 084204 (2009).
Yu, M. & Trinkle, D. R. Accurate and efficient algorithm for Bader charge integration. J. Chem. Phys. 134, 064111 (2011).
Suntivich, J., May, K. J., Gasteiger, H. A., Goodenough, J. B. & Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 334, 1383–1385 (2011).
Zhao, Y. et al. Stable iridium dinuclear heterogeneous catalysts supported on metal-oxide substrate for solar water oxidation. Proc. Natl Acad. Sci. USA 115, 2902–2907 (2018).
Zhao, Y. et al. End-on bound iridium dinuclear heterogeneous catalysts on WO3 for solar water oxidation. ACS Cent. Sci. 4, 1166–1172 (2018).
Sengodan, S. et al. Layered oxygen-deficient double perovskite as an efficient and stable anode for direct hydrocarbon solid oxide fuel cells. Nat. Mater. 14, 205–209 (2015).
Bai, S. et al. Highly active and selective hydrogenation of CO2 to ethanol by ordered Pd–Cu nanoparticles. J. Am. Chem. Soc. 139, 6827–6830 (2017).
Dong, W., Kresse, G., Furthmüller, J. & Hafner, J. Chemisorption of H on Pd(111): An ab initio approach with ultrasoft pseudopotentials. Phys. Rev. B 54, 2157–2166 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Dholabhai, P. P., Adams, J. B., Crozier, P. & Sharma, R. Oxygen vacancy migration in ceria and Pr-doped ceria: a DFT + U study. J. Chem. Phys. 132, 094104 (2010).
Wang, W. et al. Enhanced carbon dioxide electrolysis at redox manipulated interfaces. Nat. Commun. 10, 1550 (2019).
Makov, G. & Payne, M. C. Periodic boundary conditions in ab initio calculations. Phys. Rev. B 51, 4014–4022 (1995).
Neugebauer, J. & Scheffler, M. Adsorbate-substrate and adsorbate-adsorbate interactions of Na and K adlayers on Al (111). Phys. Rev. B 46, 16067 (1992).
Acknowledgements
This work was supported by the Idaho National Laboratory Laboratory Directed Research & Development Program under the Department of Energy Idaho Operations Office Contract DE-AC07-051D14517. This research made use of Idaho National Laboratory computing resources which are supported by the Office of Nuclear Energy of the U.S. Department of Energy and the Nuclear Science User Facilities under Contract No. DE-AC07-05ID14517. Sandia National Laboratories is a multimission laboratory managed and operated by National Technology & Engineering Solutions of Sandia, LLC, a wholly owned subsidiary of Honeywell International Inc., for the U.S. Department of Energy’s National Nuclear Security Administration under contract DE-NA0003525.This paper describes objective technical results and analysis. Any subjective views or opinions that might be expressed in the paper do not necessarily represent the views of the U.S. Department of Energy or the United States Government.
Author information
Authors and Affiliations
Contributions
D.D. conceived, designed and supervised the project. M.L. performed the ab initio calculations and proposed the catalyst design concept. M.L. and B.H. developed the catalysts, integrated the electrolysers, performed the characterizations, conducted electrochemical tests and analysed the data. L.-C.W. helped with the GC–MS, H2-TPR and DRIFTS tests. W.W. helped with the cell fabrication. Y.D. and J.D.S. performed the scanning transmission electron microscopy imaging, STEM–EDX and STEM–EELS. J.L. helped with the theoretical calculations and the interpretation. M.L. and B.H. wrote the manuscript and all authors contributed to the revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Catalysis thanks Michal Bajdich and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Tables 1–9, Figs. 1–47 and Note 1.
Supplementary Data 1
Atomic coordinates of the most stable Irn clusters (n = 5, 6, 11, 15).
Supplementary Data 2
Atomic coordinates of SDC (110)/Irn and (111)/Irn (n = 1, 5, 6, 11, 15) models used in DFT calculations.
Supplementary Data 3
Atomic coordinates of the initial and final configurations of the trajectories in AIMD simulations.
Source data
Source Data Fig. 2
Gibbs free energies for the CO2 hydrogenation reaction calculated by DFT.
Source Data Fig. 3
Characterization data of XRD patterns, HAADF line profiles, XPS spectra and EELS spectra.
Source Data Fig. 4
Electrochemical measurement data and DRIFTS spectra.
Source Data Fig. 5
Electrochemical measurement data.
Rights and permissions
About this article
Cite this article
Li, M., Hua, B., Wang, LC. et al. Switching of metal–oxygen hybridization for selective CO2 electrohydrogenation under mild temperature and pressure. Nat Catal 4, 274–283 (2021). https://doi.org/10.1038/s41929-021-00590-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-021-00590-5
This article is cited by
-
Integrated energy storage and CO2 conversion using an aqueous battery with tamed asymmetric reactions
Nature Communications (2024)
-
Advances in bio-inspired electrocatalysts for clean energy future
Nano Research (2024)
-
Recent progress in oxygen electrodes for protonic ceramic electrochemical cells
Journal of the Korean Ceramic Society (2024)
-
Lowering the operating temperature of protonic ceramic electrochemical cells to <450 °C
Nature Energy (2023)
-
Revitalizing interface in protonic ceramic cells by acid etch
Nature (2022)