Abstract
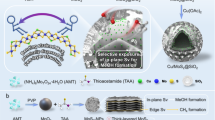
The low-temperature hydrogenation of CO2 to methanol is of great significance for the recycling of this greenhouse gas to valuable products, however, it remains a great challenge due to the trade-off between catalytic activity and selectivity. Here, we report that CO2 can dissociate at sulfur vacancies in MoS2 nanosheets to yield surface-bound CO and O at room temperature, thus enabling a highly efficient low-temperature hydrogenation of CO2 to methanol. Multiple in situ spectroscopic and microscopic characterizations combined with theoretical calculations demonstrated that in-plane sulfur vacancies drive the selective hydrogenation of CO2 to methanol by inhibiting deep hydrogenolysis to methane, whereas edge vacancies facilitate excessive hydrogenation to methane. At 180 °C, the catalyst achieved a 94.3% methanol selectivity at a CO2 conversion of 12.5% over the in-plane sulfur vacancy-rich MoS2 nanosheets, which notably surpasses those of previously reported catalysts. This catalyst exhibited high stability for over 3,000 hours without any deactivation, rendering it a promising candidate for industrial application.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information or from the corresponding authors upon reasonable request. The atomic positions of the reaction intermediates are available in the figshare repository (https://doi.org/10.6084/m9.figshare.13498254.v1).
References
Aresta, M., Dibenedetto, A. & Angelini, A. Catalysis for the valorization of exhaust carbon: from CO2 to chemicals, materials, and fuels. Technological use of CO2. Chem. Rev. 114, 1709–1742 (2014).
Song, C. S. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 115, 2–32 (2006).
Goeppert, A. et al. Recycling of carbon dioxide to methanol and derived products – closing the loop. Chem. Soc. Rev. 43, 7995–8048 (2014).
Appel, A. M. et al. Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation. Chem. Rev. 113, 6621–6658 (2013).
Wang, W., Wang, S., Ma, X. & Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 40, 3703–3727 (2011).
Kondratenko, E. V. et al. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 6, 3112–3135 (2013).
Yarulina, I. et al. Recent trends and fundamental insights in the methanol-to-hydrocarbons process. Nat. Catal. 1, 398–411 (2018).
Graciani, J. et al. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science 345, 546–550 (2014).
Tackett, B. M., Gomez, E. & Chen, J. G. Net reduction of CO2 via its thermocatalytic and electrocatalytic transformation reactions in standard and hybrid processes. Nat. Catal. 2, 381–386 (2019).
Wang, J. et al. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol. Sci. Adv. 3, e1701290 (2017).
Martin, O. et al. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation. Angew. Chem. Int. Ed. 55, 6261–6265 (2016).
Frei, M. S. et al. Mechanism and microkinetics of methanol synthesis via CO2 hydrogenation on indium oxide. J. Catal. 361, 313–321 (2018).
Liu, X. et al. Selective transformation of carbon dioxide into lower olefins with a bifunctional catalyst composed of ZnGa2O4 and SAPO-34. Chem. Commun. 54, 140–143 (2018).
Ni, Y. et al. Selective conversion of CO2 and H2 into aromatics. Nat. Commun. 9, 3457 (2018).
Gao, P. et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 9, 1019–1024 (2017).
Gao, P. et al. Direct production of lower olefins from CO2 conversion via bifunctional catalysis. ACS Catal. 8, 571–578 (2018).
Jung, K. D. & Bell, A. T. Role of hydrogen spillover in methanol synthesis over Cu/ZrO2. J. Catal. 193, 207–223 (2000).
Collins, S., Baltanas, M. & Bonivardi, A. An infrared study of the intermediates of methanol synthesis from carbon dioxide over Pd/β-GaO. J. Catal. 226, 410–421 (2004).
Conner, W. C. Jr & Falconer, J. L. Spillover in heterogeneous catalysis. Chem. Rev. 95, 759–788 (1995).
Rui, N. et al. CO2 hydrogenation to methanol over Pd/In2O3: effects of Pd and oxygen vacancy. Appl. Catal. B 218, 488–497 (2017).
Yin, Y. Z. et al. Pd@zeolitic imidazolate framework-8 derived PdZn alloy catalysts for efficient hydrogenation of CO2 to methanol. Appl. Catal. B 234, 143–152 (2018).
Kattel, S., Liu, P. & Chen, J. G. Tuning selectivity of CO2 hydrogenation reactions at the metal/oxide interface. J. Am. Chem. Soc. 139, 9739–9754 (2017).
Fisher, I. A. & Bell, A. T. In-situ infrared study of methanol synthesis from H2/CO2 over Cu/SiO2 and Cu/ZrO2/SiO2. J. Catal. 172, 222–237 (1997).
Studt, F. et al. Discovery of a Ni-Ga catalyst for carbon dioxide reduction to methanol. Nat. Chem. 6, 320–324 (2014).
Behrens, M. et al. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 336, 893–897 (2012).
Kuld, S. et al. Quantifying the promotion of Cu catalysts by ZnO for methanol synthesis. Science 352, 969–974 (2016).
Behrens, M. Heterogeneous catalysis of CO2 conversion to methanol on copper surfaces. Angew. Chem. Int. Ed. 53, 12022–12024 (2014).
Zabilskiy, M. et al. The unique interplay between copper and zinc during catalytic carbon dioxide hydrogenation to methanol. Nat. Commun. 11, 2409 (2020).
Sehested, J. Industrial and scientific directions of methanol catalyst development. J. Catal. 371, 368–375 (2019).
Behrens, M. Promoting the synthesis of methanol: understanding the requirements for an industrial catalyst for the conversion of CO2. Angew. Chem. Int. Ed. 55, 14906–14908 (2016).
Schlögl, R. Heterogeneous catalysis. Angew. Chem. Int. Ed. 54, 3465–3520 (2015).
Liu, G. et al. MoS2 monolayer catalyst doped with isolated Co atoms for the hydrodeoxygenation reaction. Nat. Chem. 9, 810–816 (2017).
Cai, L. et al. Vacancy-induced ferromagnetism of MoS2 nanosheets. J. Am. Chem. Soc. 137, 2622–2627 (2015).
Chianelli, R. R., Daage, M. & Ledoux, M. J. Fundamental studies of transition-metal sulfide catalytic materials. Adv. Catal. 40, 177–232 (1994).
Okamoto, Y. et al. Structure of the active sites of Co–Mo hydrodesulfurization catalysts as studied by magnetic susceptibility measurement and NO adsorption. J. Phys. Chem. B 109, 288–296 (2005).
Weber, T. et al. Basic reaction steps in the sulfidation of crystalline MoO3 to MoS2 as studied by X-ray photoelectron and infrared emission spectroscopy. J. Phys. Chem. 100, 14144–14150 (1996).
Peng, Y.-K. et al. Trimethylphosphine-assisted surface fingerprinting of metal oxide nanoparticle by 31P solid-state NMR: a zinc oxide case study. J. Am. Chem. Soc. 138, 2225–2234 (2016).
Zhou, J. et al. A library of atomically thin metal chalcogenides. Nature 556, 355–359 (2018).
Raybaud, P. et al. Ab initio study of the H2–H2S/MoS2 gas–solid interface: the nature of the catalytically active sites. J. Catal. 189, 129–146 (2000).
Tsai, C., Abild-Pedersen, F. & Norskov, J. K. Tuning the MoS2 edge-site activity for hydrogen evolution via support interactions. Nano Lett. 14, 1381–1387 (2014).
Larmier, K. et al. CO2-to-methanol hydrogenation on zirconia-supported copper nanoparticles: reaction intermediates and the role of the metal-support interface. Angew. Chem. Int. Ed. 56, 2318–2323 (2017).
Kunkes, E. L. et al. Hydrogenation of CO2 to methanol and CO on Cu/ZnO/Al2O3: is there a common intermediate or not? J. Catal. 328, 43–48 (2015).
Medford, A. J. et al. CatMAP: a software package for descriptor-based microkinetic mapping of catalytic trends. Catal. Lett. 145, 794–807 (2015).
Jaramillo, T. F. et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 317, 100–102 (2007).
Hinnemann, B. et al. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 127, 5308–5309 (2005).
Byskov, L. S., Nørskov, J. K., Clausen, B. S. & Topsøe, H. DFT calculations of unpromoted and promoted MoS2-based hydrodesulfurization catalysts. J. Catal. 187, 109–122 (1999).
Prins, R., De Beer, V. H. J. & Somorjai, G. A. Structure and function of the catalyst and the promoter in Co–Mo hydrodesulfurization catalysts. Catal. Rev. 31, 1–41 (1989).
Duyar, M. S. et al. A highly active molybdenum phosphide catalyst for methanol synthesis from CO and CO2. Angew. Chem. Int. Ed. 57, 15045–15050 (2018).
Posada-Pérez, S. et al. The bending machine: CO2 activation and hydrogenation on δ-MoC(001) and β-Mo2C(001) surfaces. Phys. Chem. Chem. Phys. 16, 14912–14921 (2014).
Deng, J. et al. Triggering the electrocatalytic hydrogen evolution activity of the inert two-dimensional MoS2 surface via single-atom metal doping. Energy Environ. Sci. 8, 1594–1601 (2015).
Wang, S., Goulas, K. & Iglesia, E. Condensation and esterification reactions of alkanals, alkanones, and alkanols on TiO2: elementary steps, site requirements, and synergistic effects of bifunctional strategies. J. Catal. 340, 302–320 (2016).
Chinchen, G., Hay, C., Vandervell, H. & Waugh, K. The measurement of copper surface areas by reactive frontal chromatography. J. Catal. 103, 79–86 (1987).
Luo, L. et al. Gas-phase reaction network of Li/MgO-catalyzed oxidative coupling of methane and oxidative dehydrogenation of ethane. ACS Catal. 9, 2514–2520 (2019).
Zhou, Z. et al. The vacuum ultraviolet beamline/endstations at NSRL dedicated to combustion research. J. Synchrotron Radiat. 23, 1035–1045 (2016).
Li, Y. Y. et al. Disclosure of the surface composition of TiO2-supported gold palladium bimetallic catalysts by high-sensitivity low-energy ion scattering spectroscopy. ACS Catal. 8, 1790–1795 (2018).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Kresse, G. & Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996); erratum 78, 1396 (1997).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Larsen, A. H. et al. The atomic simulation environment—a Python library for working with atoms. J. Phys. Condens. Matter 29, 273002 (2017).
Studt, F. et al. The mechanism of CO and CO2 hydrogenation to methanol over Cu-based catalysts. ChemCatChem 7, 1105–1111 (2015).
Peacock-López, E. & Suhl, H. Compensation effect in thermally activated processes. Phys. Rev. B 26, 3774–3782 (1982).
McCoy, B. J. Compensation effect in the dissociation of anharmonic oscillators: a model for catalysis. J. Chem. Phys. 80, 3629–3631 (1984).
Bond, G. C., Keane, M. A., Kral, H. & Lercher, J. A. Compensation phenomena in heterogeneous catalysis: general principles and a possible explanation. Catal. Rev. 42, 323–383 (2000).
Pickard, C. J. & Mauri, F. All-electron magnetic response with pseudopotentials: NMR chemical shifts. Phys. Rev. B 63, 245101 (2001).
Yates, J. R., Pickard, C. J. & Mauri, F. Calculation of NMR chemical shifts for extended systems using ultrasoft pseudopotentials. Phys. Rev. B 76, 024401 (2007).
Thrane, J. et al. Methanol-assisted autocatalysis in catalytic methanol synthesis. Angew. Chem. Int. Ed. 59, 18189–18193 (2020).
Gao, P. et al. Influence of Zr on the performance of Cu/Zn/Al/Zr catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol. J. Catal. 298, 51–60 (2013).
Tisseraud, C. et al. The Cu–ZnO synergy in methanol synthesis from CO2, part 2: origin of the methanol and CO selectivities explained by experimental studies and a sphere contact quantification model in randomly packed binary mixtures on Cu–ZnO coprecipitate catalysts. J. Catal. 330, 533–544 (2015).
van den Berg, R. et al. Support functionalization to retard Ostwald ripening in copper methanol synthesis catalysts. ACS Catal. 5, 4439–4448 (2015).
van den Berg, R. et al. Structure sensitivity of Cu and CuZn catalysts relevant to industrial methanol synthesis. Nat. Commun. 7, 13057 (2016).
Boudart, M. Turnover rates in heterogeneous catalysis. Chem. Rev. 95, 661–666 (1995).
Deng, J. et al. High-performance hydrogen evolution electrocatalysis by layer-controlled MoS2 nanosheets. RSC Adv. 4, 34733–34738 (2014).
Zeng, T. et al. Density functional theory study of CO adsorption on molybdenum sulfide. J. Phys. Chem. B 109, 2846–2854 (2005).
Travert, A. et al. Parallel between infrared characterisation and ab initio calculations of CO adsorption on sulphided Mo catalysts. Catal. Today 70, 255–269 (2001).
Uvdal, P., Weldon, M. K. & Friend, C. M. Adsorbate symmetry and Fermi resonances of methoxide adsorbed on Mo(110) as studied by surface infrared spectroscopy. Phys. Rev. B 50, 12258–12261 (1994).
Guyon, P. M., Chupka, W. A. & Berkowitz, J. Photoionization mass spectrometric study of formaldehyde H2CO, HDCO and D2CO. J. Chem. Phys. 64, 1419–1436 (1976).
Berkowitz, J. Photoionization of CH3OH, CD3OH, and CH3OD: dissociative ionization mechanisms and ionic structures. J. Chem. Phys. 69, 3044–3054 (1978).
Acknowledgements
We acknowledge the financial support from the Ministry of Science and Technology of China (Nos. 2016YFA0204100, 2016YFA0200200, 2017YFA0402800 and 2017YFB0602201), the National Natural Science Foundation of China (Nos. 21890753, 21988101, 91545203 and 21433008), the Key Research Program of Frontier Sciences of the Chinese Academy of Sciences (No. QYZDB-SSW-JSC020), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB36030200) and the DNL Cooperation Fund, CAS (No. DNL180201). We thank the staff at the BL14W1 beamline of the Shanghai Synchrotron Radiation Facilities for assistance with the EXAFS and XANES measurements. We also thank H. Su for assistance in the DFT calculations.
Author information
Authors and Affiliations
Contributions
D.D. and Ye W. conceived and designed the experiments. J.H. performed the materials synthesis, characterization and performance experiments. L.Y. contributed to the DFT calculations. J.D. assisted with the materials synthesis. Yong W., K.C., Q.Z. and R.H. assisted with data analysis and manuscript revision. C.M. conducted the HAADF-STEM experiments. S.Z. assisted with the HRTEM experiments. W.W., S.Y., Y.P., J.Y., H.M. and F.Q. conducted the SVUV-PIMS experiments. Yongke W., Y.Z. and M.-S.C. assisted with the XPS and HS-LEIS experiments. Z.Z., G.H. and X.H. conducted the NMR experiments and analysed the data. X.M. and R.H. assisted with the in situ EPR experiments. Q.J. assisted with the NO adsorption experiments. J.M. assisted with the O2 adsorption experiments. X.B. provided constructive suggestions. J.H., L.Y., Ye W. and D.D. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Catalysis thanks Jose Rodriguez and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Structural stability, reproducibility and durability of the FL-MoS2 catalyst.
a–h, TEM images of the fresh (a–c) and used FL-MoS2 (after 3000 hours of durability test, e–g). HAADF-STEM images of the fresh FL-MoS2 (d) and the used FL-MoS2 (h) with the corresponding FFT patterns (inside). Both the fresh and the used FL-MoS2 exhibit highly crystallized 2H-MoS2 structures as reflected by the FFT patterns. i,j, Layer number statistics for at least 100 of MoS2 layers of the fresh FL-MoS2 (i) and the used FL-MoS2 after 3000 hours of durability test (j) based on TEM results. k, XRD pattern of the fresh and used FL-MoS2. l, Equilibrium conversion of CO2 as a function of temperature in the hydrogenation of CO2 to methanol. Total pressure is 50 bar with H2/CO2 ratio of 3. m, Reproducibility test of individually synthesized FL-MoS2 catalysts. Catalysts were pretreated in-situ by H2 at 300 °C for 3 hours. Reaction activity tests were performed at 3000 mL gcat.−1 h−1, 180 °C, 50 bar and H2/CO2 ratio of 3:1. n, Durability test of the FL-MoS2 for CO2 hydrogenation at 260 °C. Catalyst was pretreated in-situ by H2 at 300 °C for 3 hours. Reaction activity test was performed at 15000 mL gcat.−1 h−1, 260 °C, 50 bar and H2/CO2 ratio of 3:1.
Extended Data Fig. 2 Comparison in catalytic performances of the FL-MoS2 and the Cu/ZnO/Al2O3 (CuZnAl) and other catalysts.
a, Comparison in net STYmethanol over the Cu/ZnO/Al2O3 (CuZnAl) and the FL-MoS2 at 60 bar (Supplementary Table 1 for more details, # represents data of this work). b, Comparison in the forward TOF for methanol formation (calculated on the basis of the amount of exposed Cu, Mo or Sv sites) over the Cu/ZnO/Al2O3 (CuZnAl) and the FL-MoS2 at 50 bar and iso-conversion levels. It was reported that for the Cu/ZnO/Al2O3 catalyst only small fractions of surface synergetic sites are considered to be highly active for the CO2 conversion.25,26,27,28,29,30 However, the real high-active sites can hardly be precisely quantified in the actual systems owing to the difficulty in identifying them based on the currently reported characterization techniques. Thus, in the calculation of the TOF, all surface Cu atoms are included in the amount of the active sites, as typically done in previous works74,75,76,77,78. The TOF on the Cu/ZnO/Al2O3 catalyst calculated in this way should represent an average activity level of the active sites31,79. c, Comparison in the net TOF for methanol formation over the FL-MoS2 and other state-of-the-art catalysts calculated on the basis of the amount of exposed metal (Supplementary Table 2 for more details, the order of catalysts in (c) corresponding to that in Supplementary Table 2). Reaction activity tests of the FL-MoS2 were performed at 50 bar with a GHSV of 36000 mL gcat.−1 h−1. d, The catalytic performances of the Cu/ZnO/Al2O3 (CuZnAl) and the FL-MoS2 under identical reaction conditions, which were tested at 180 °C, 50 bar and 3000 mL gcat.−1 h−1. e, Selectivity to methanol as a function of CO2 conversion over the FL-MoS2 and the Cu/ZnO/Al2O3 (CuZnAl) obtained by varying the GHSV in the range of 3000 to 36000 mL gcat.−1 h−1 at 50 bar and different temperatures. Catalysts were pretreated in-situ by H2 at 300 °C for 3 hours. Reaction activity tests were performed using a tubular fixed-bed reactor with a H2/CO2 ratio of 3. TOFs of the FL-MoS2 were calculated on the basis of NO adsorption capacities.
Extended Data Fig. 3 Characterizations of the FL-MoS2 at different reaction stages.
a, In-situ EPR spectra of the fresh, in-situ reduced FL-MoS2 and the FL-MoS2 after in-situ reaction for 4 hours, respectively. Before measurements, the FL-MoS2 was treated in-situ by H2 (denoted as FL-MoS2-reduced), reaction gas (H2/CO2 = 3/1, denoted as FL-MoS2-used) and purged with He without exposure to the air (see Methods for more details). b, Variation of the Mo-S coordination numbers of the FL-MoS2 depending on the temperature during the H2 pretreatment process. Debye-Waller factor (σ2) = 0.0041 ± 0.0002 Å2, inner potential correction (∆E0) = 2.7 ± 0.2 eV. Results show that the Mo-S coordination number decreases as the reduction temperature increases, which may be caused by the loss of sulphur atoms during H2 treatment. c, d, XPS spectra of the fresh, reduced and used FL-MoS2, respectively. e, S/Mo atomic ratios in the fresh, reduced and used FL-MoS2, which were calculated based on the sensitivity factor and the peak area of the Mo 3d and S 2p, showing a decreasing trend.
Extended Data Fig. 4 Structure-activity relationship of different MoS2 catalysts.
a–d, HRTEM images of the FL-MoS2 (a), ML-MoS2 (b), TL-MoS2 (c) and Bulk-MoS2 (d), respectively. e–g, Layer number statistics for at least 100 of MoS2 layers of the FL-MoS2 (e), ML-MoS2 (f) and TL-MoS2 (g), respectively, based on the HRTEM images. h, XRD patterns of different MoS2 catalysts. These MoS2 catalysts exhibit a typical hexagonal 2H-MoS2 crystal, corresponding to PDF #37-1492. i, Correlations between the intensity ratio of the XRD (002) and (100) peaks (I(002)/(100)) and the layer thicknesses of different MoS2 catalysts. Error bars represent the standard deviation for statistical layer numbers of different MoS2 catalysts, respectively. The I(002)/(100) of MoS2 had been reported to assess the variation of layer numbers in a series of MoS2 materials80. The increasing trend of I(002)/(100) values of the FL-MoS2 (0.78), ML-MoS2 (1.12), TL-MoS2 (1.76) and Bulk-MoS2 (8.77) indicate the increasing layer numbers of these MoS2 catalysts. j, In-situ EPR spectra of H2-pretreated MoS2 samples (see Methods for more details). k, The correlation between the rf for methanol formation and the density of single electron for the MoS2 catalysts. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) was used as an external standard to estimate the density of sulphur vacancies. l, BET surface area of different MoS2 samples. m, The correlation between the rf for methanol formation and the BET surface area of the MoS2 catalysts. n, The 31P MAS NMR spectra of in-situ H2-pretreated MoS2 samples after full adsorption of TMP. The black lines are raw data and red lines are fitting results. o, The correlation between the rf for methanol formation and the specific adsorption capacity of TMP for the MoS2 catalysts. p, CO2 hydrogenation performances and TMP adsorption capacities of different MoS2 catalysts. Catalysts were pretreated in-situ by H2 at 300 °C for 3 hours. Reaction activity tests were performed at 3000 mL gcat.−1 h−1, 180 °C, 50 bar and H2/CO2 ratio of 3:1.
Extended Data Fig. 5 Characterizations of the structures and catalytic performances of the FL-MoS2 and the NP-MoS2.
a,b, TEM images of the fresh FL-MoS2. c,d, TEM images of the fresh NP-MoS2. e, XRD patterns of the fresh FL-MoS2 and NP-MoS2. These MoS2 catalysts exhibit a typical hexagonal 2H-MoS2 crystal, corresponding to PDF #37-1492. f, Adsorption capacity of O2 over the reduced FL-MoS2 and NP-MoS2. g,h, Selectivity of methanol and methane as a function of CO2 conversion over the FL-MoS2 and the NP-MoS2 obtained by varying the GHSV at different temperatures. Catalysts were pretreated in-situ by H2 at 300 °C for 3 hours. Reaction activity tests were performed using a tubular fixed-bed reactor at 50 bar and H2/CO2 of 3. i, Formation rate of CH4 from methanol decomposition over the FL-MoS2 and NP-MoS2 after H2-pretreatment. Liquid methanol (0.01 mL min−1) and high purity H2 with 5% Ar with a gas flow rate of 30 mL min−1 were simultaneously fed into the tubular fixed-bed reactor loaded with 200 mg catalyst at 1 bar and 180 °C.
Extended Data Fig. 6 Optimized structures of adsorbed TMP and NO, and the chemical shifts of 31P of TMP molecules at the edge and basal plane.
a, Optimized structures of adsorbed TMP at the edge and basal plane, respectively. The edge double-Sv and triple-Sv sites are fully covered by three and four TMP molecules, respectively, with differential adsorption free energies (Gads) of −0.39 and −0.38 eV at 100% coverage. The in-plane double-Sv and triple-Sv sites can adsorb only one TMP molecule with Gads of −0.29 and −0.18 eV, respectively. b, DFT-calculated chemical shifts of 31P of TMP molecules adsorbed at the double-Sv and triple-Sv sites of MoS2 edges and basal plane. The chemical shift of H3PO4 was used as the reference, and the calculated chemical shift of (NH4)2HPO4 was corrected by subtracting 1.13 ppm (the experimental chemical shift of (NH4)2HPO4 is 1.13 ppm relative to that of H3PO4). c, DFT-calculated adsorption structures of NO at the in-plane and Mo-edge S vacancies.
Extended Data Fig. 7 DFT calculation models and optimized structures.
a, b, A nanoribbon (a) and a tri-layer (b) model of MoS2 for simulating the edge and in-plane S vacancies. The dotted red circles denote the S atoms to be removed for creating the vacancies. c, Models of single, double and triple S vacancies at the edge and basal plane of MoS2, denoted as single-Sv, double-Sv, and triple-Sv, respectively. The dotted red circles denote the S vacancies. d, Comparison between the formation energies of Mo-edge and S-edge S vacancies. For the S-edge, the first stage denotes removal of one S atom from the terminating S dimer. e, f, Optimized structures of the in-plane and Mo-edge S-H species (e) and the S vacancies (Sv) at the brim, Mo-edge, and corner (f).
Extended Data Fig. 8 DFT studies of the reaction mechanisms of CO2 hydrogenation over the triple-Sv of MoS2.
a, b, Free energy diagram of the CO2 hydrogenation reaction pathways on the in-plane and Mo-edge triple-Sv, respectively. Insets show the atomic structures of the S vacancies and the reaction intermediates. The red dotted circles in structures 1 denote the positions of S vacancies. c, d, Turnover frequencies (TOFs) for the generation of CH3OH, CO and CH4 from the micro-kinetics modelling of the reaction mechanisms at the in-plane (c) and Mo-edge (d) triple-Sv, respectively.
Extended Data Fig. 9 Identification of the CO2 hydrogenation mechanism.
a, b, In-situ DRIFT spectra of CO2 dissociation over the FL-MoS2 (a) and the NP-MoS2 (b) at 25 °C. As H2 was switched to CO2, the linearly absorbed CO species (~2078 cm−1, a, b)81,82, the Mo=O species (peaks at 900 ~ 960 cm−1, a, b) and the Mo-O-Mo species (broad band at around 700 ~ 860 cm−1, a)36 were observed, indicating that the coordinatively unsaturated Mo can easily dissociate CO2 to *CO and *O at 25 °C. c, In-situ DRIFTS of the CO2 hydrogenation and then H2 reduction process at 25 °C. The reduced FL-MoS2 catalyst was exposed to CO2/H2 (1/3) reaction gas in a gas flow of 30 mL min−1. The formation of *CO and O* species36,81,82 were observed. With the increase of reaction time, the peaks in the range from 2800-3000 cm−1 grew up, which were attributed to the CH3O* species83. When switching gas flow from the reaction gas to pure H2, the CO* disappeared and the CH3O* and O* decreased gradually. d, In-situ DRIFTS of the H2 reduction process with increasing temperatures. As the temperature increases from 25 to 300 °C, the O* species can be gradually removed by the H2. e, In-situ DRIFT spectra of CO2 hydrogenation over the FL-MoS2 catalyst at 30 bar and 180 °C with a CO2/H2 ratio of 1/3. f, In-situ DRIFT spectra of CO2 dissociation over the FL-MoS2 at 180 °C and 30 bar. g, In-situ DRIFT spectra of the FL-MoS2 after switching the feed gas from CO2 to H2 at 180 °C and 30 bar. The line segments denote the scale bars of the absorbance. h, In-situ Mo K-edge XANES of the FL-MoS2. After the first spectrum of the fresh FL-MoS2 catalyst was obtained, the catalyst was treated in-situ by H2 at 300 °C for 3 hours. Then, the catalyst was cooled down to 180 °C under H2 atmosphere, and the second spectrum was obtained. After that, the catalyst was exposed to CO2 in a gas flow of 30 mL min−1, and the third spectrum was obtained. Subsequently, the feeding gas was switched to the reaction gas (H2/CO2 = 3/1) with a gas flow of 30 mL min−1, and the fourth spectrum was obtained. i, In-situ SVUV-PIMS detection of gas-phase products during the CO2 hydrogenation process. j, Photoionization efficiency spectra of the signals of m/z 30 and 32 during the CO2 hydrogenation process. The FL-MoS2 catalyst was pretreated in-situ by H2 at 300 °C for 1 hour. After that, CO2/H2 (1/3) was led through the reduced FL-MoS2 catalyst at 180 °C and 5 bar. The signals of m/z 30 and 32 give ionization thresholds of 10.87 and 10.85 eV that agree well with the ionization thresholds of formaldehyde (HCHO) and methanol (CH3OH)84,85, respectively, demonstrating the existence of HCHO species during the CO2 hydrogenation reaction.
Extended Data Fig. 10 DFT studies of the dissociation of methanol in H2 on the S vacancies of MoS2.
a, c, The dissociation reaction mechanisms of methanol in H2 on the in-plane and Mo-edge double-Sv (a) and triple-Sv (c), respectively. Insets show the structures of the reaction intermediates. Dotted red circles denote the S vacancies. b, d, Turnover frequencies (TOFs) for the generation of methane from the micro-kinetics modelling of the reaction mechanisms at the double-Sv (b) and triple-Sv (d), respectively.
Supplementary information
Supplementary Information
Supplementary Methods, Tables 1–3 and references.
Rights and permissions
About this article
Cite this article
Hu, J., Yu, L., Deng, J. et al. Sulfur vacancy-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol. Nat Catal 4, 242–250 (2021). https://doi.org/10.1038/s41929-021-00584-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-021-00584-3
This article is cited by
-
Investigating the role of undercoordinated Pt sites at the surface of layered PtTe2 for methanol decomposition
Nature Communications (2024)
-
A Ce-CuZn catalyst with abundant Cu/Zn-OV-Ce active sites for CO2 hydrogenation to methanol
Nature Communications (2024)
-
Self-propelled assembly of nanoparticles with self-catalytic regulation for tumour-specific imaging and therapy
Nature Communications (2024)
-
Upgrading CO2 to sustainable aromatics via perovskite-mediated tandem catalysis
Nature Communications (2024)
-
Unraveling the evolution of oxygen vacancies in TiO2−x/Cu and its role in CO2 hydrogenation
Science China Chemistry (2024)