Abstract
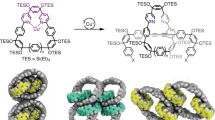
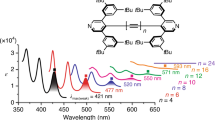
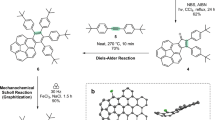
The discoveries of new forms of carbon have always opened doors to new science and technology. In 1991, three-dimensional (3D) periodic carbon crystals with negative Gaussian curvatures that consist of six- and eight-membered rings were proposed. To realize these 3D periodic carbon crystals (known as Mackay crystals), methods for creating polyaromatic structures embedding eight-membered rings must be developed. Here we report two annulative coupling reactions that form an eight-membered ring through catalytic C–H functionalization. We have discovered that bay-chlorinated polyaromatics undergo either annulative dimerization or cross-coupling with biphenylene in the presence of a palladium catalyst to form various hitherto inaccessible polyaromatics embedding an eight-membered ring. The threefold annulative cross-coupling of 1,5,9-trichlorotriphenylene allowed construction of a highly curved nanocarbon. The present work not only demonstrates the potential of annulative coupling for constructing octagonal nanocarbons but also provides a conceptual pathway for the synthetic realization of 3D periodic carbon crystals.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Materials and methods, experimental procedures, detailed optimization studies, mechanistic studies, photophysical studies and NMR spectra are available in the Supplementary Information or from the corresponding authors upon reasonable request. The atomic coordinates of the optimized models are provided in Supplementary Data 1. Crystallographic data for compounds 5d, 5e and 8a are available free of charge from the Cambridge Crystallographic Data Centre under deposition numbers 1898108, 1898109 and 1898106. Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Kroto, H. W., Heath, J. R., O’Brien, S. C., Curl, R. F. & Smalley, R. E. C60: buckminsterfullerene. Nature 318, 162–163 (1985).
Iijima, S. Helical microtubules of graphitic carbon. Nature 354, 56–58 (1991).
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Segawa, Y., Ito, H. & Itami, K. Structurally uniform and atomically precise carbon nanostructures. Nat. Rev. Mater 1, 15002 (2016).
Mackay, A. L. & Terrones, H. Diamond from graphite. Nature 352, 762–762 (1991).
Lenosky, T., Gonze, X., Teter, M. & Elser, V. Energetics of negatively curved graphitic carbon. Nature 355, 333–335 (1992).
Park, N. et al. Magnetism in all-carbon nanostructures with negative Gaussian curvature. Phys. Rev. Lett. 91, 237204 (2003).
Tagami, M., Liang, Y., Naito, H., Kawazoe, Y. & Kotani, M. Negatively curved cubic carbon crystals with octahedral symmetry. Carbon 76, 266–274 (2014).
Majewski, M. A. & Stępień, M. Bowls, hoops, and saddles: synthetic approaches to curved aromatic molecules. Angew. Chem. Int. Ed. 58, 86–116 (2019).
Pun, S. H. & Miao, Q. Toward negatively curved carbons. Acc. Chem. Res. 51, 1630–1642 (2018).
Deng, C.-L., Peng, X.-S., Wong, H. N. C. in Polycyclic Arenes and Heteroarenes: Synthesis, Properties and Applications (ed. Miao, Q.) 111–141 (VCH-Wiley, 2016).
Han, J.-W., Peng, X.-S. & Wong, H. N. C. Synthesis of tetraphenylene derivatives and their recent advances. Natl Sci. Rev. 4, 892–916 (2017).
Rickhaus, M., Mayor, M. & Juríček, M. Chirality in curved polyaromatic systems. Chem. Soc. Rev. 46, 1643–1660 (2017).
Márquez, I. R., Castro-Fernández, S., Millán, A. & Campaña, A. G. Synthesis of distorted nanographenes containing seven- and eight-membered carbocycles. Chem. Commun. 54, 6705–6718 (2018).
Thulin, B. & Wennerström, O. Synthesis of [2.0.2.0]metacyclophanediene and bi-4,5-phenanthrylene. Tetrahedron Lett. 18, 929–930 (1977).
Leach, D. N. & Reiss, J. A. Cyclophanes. 9. Dibenzo[def,pqr]tetraphenylene: a benzoannulated cyclooctatetraene composed of orthogonal aromatic systems. J. Org. Chem. 43, 2484–2487 (1978).
Sakamoto, Y. & Suzuki, T. Tetrabenzo[8]circulene: aromatic saddles from negatively curved graphene. J. Am. Chem. Soc. 135, 14074–14077 (2013).
Cheung, K. Y., Chan, C. K., Liu, Z. & Miao, Q. A twisted nanographene consisting of 96 carbon atoms. Angew. Chem. Int. Ed. 56, 9003–9007 (2017).
Pun, S. H. et al. Synthesis, structures, and properties of heptabenzo[7]circulene and octabenzo[8]circulene. J. Am. Chem. Soc. 141, 9680–9686 (2019).
Miller, R. W., Duncan, A. K., Schneebeli, S. T., Gray, D. L. & Whalley, A. C. Synthesis and structural data of tetrabenzo[8]circulene. Chem. Eur. J. 20, 3705–3711 (2014).
Miller, R. W., Averill, S. E., Van Wyck, S. J. & Whalley, A. C. General method for the synthesis of functionalized tetrabenzo[8]circulenes. J. Org. Chem. 81, 12001–12005 (2016).
Feng, C.-N., Kuo, M.-Y. & Wu, Y.-T. Synthesis, structural analysis, and properties of [8]circulenes. Angew. Chem. Int. Ed. 52, 7791–7794 (2013).
Feng, C.-N., Hsu, W.-C., Li, J.-Y., Kuo, M.-Y. & Wu, Y.-T. Per-substituted [8]circulene and its non-planar fragments: synthesis, structural analysis, and properties. Chem. Eur. J. 22, 9198–9208 (2016).
Ito, H., Ozaki, K. & Itami, K. Annulative π-extension (APEX): rapid access to fused arenes, heteroarenes, and nanographenes. Angew. Chem. Int. Ed. 56, 11144–11164 (2017).
Ito, H., Segawa, Y., Murakami, K. & Itami, K. Polycyclic arene synthesis by annulative π-extension. J. Am. Chem. Soc. 141, 3–10 (2019).
Koga, Y., Kaneda, T., Saito, Y., Murakami, K. & Itami, K. Synthesis of partially and fully fused polyaromatics by annulative chlorophenylene dimerization. Science 359, 435–439 (2018).
Rajca, A., Safronov, A., Rajca, S. & Shoemaker, R. Double helical octaphenylene. Angew. Chem. Int. Ed. 36, 488–491 (1997).
Zhen, Y. et al. Chiral nanoribbons based on doubly-linked oligo-perylene bisimides. Chem. Commun. 46, 6078–6080 (2010).
Chen, J.-X., Han, J.-W. & Wong, H. N. C. Synthesis and chiroptical properties of double-helical (M)- and (P)-o-oligophenylenes. Org. Lett. 17, 4296–4299 (2015).
Mouri, K., Saito, S. & Yamaguchi, S. Highly flexible π-expanded cyclooctatetraenes: cyclic thiazole tetramers with head-to-tail connection. Angew. Chem. Int. Ed. 51, 5971–5975 (2012).
Jiang, H., Zhang, Y., Chen, D., Zhou, B. & Zhang, Y. An approach to tetraphenylenes via Pd-catalyzed C−H functionalization. Org. Lett. 18, 2032–2035 (2016).
Zhu, C., Zhao, Y., Wang, D., Sun, W.-Y. & Shi, Z. Palladium-catalyzed direct arylation and cyclization of o-iodobiaryls to a library of tetraphenylenes. Sci. Rep. 6, 33131 (2016).
Fukuzumi, K., Nishii, Y. & Miura, M. Palladium-catalyzed synthesis of heteroarene-fused cyclooctatetraenes through dehydrogenative cyclodimerization. Angew. Chem. Int. Ed. 56, 12746–12750 (2017).
Edelbach, B. L., Lachicotte, R. J. & Jones, W. D. Mechanistic investigation of catalytic carbon–carbon bond activation and formation by platinum and palladium phosphine complexes. J. Am. Chem. Soc. 120, 2843–2853 (1998).
Masselot, D., Charmant, J. P. H. & Gallagher, T. Intercepting palladacycles derived by C–H insertion. A mechanism-driven entry to heterocyclic tetraphenylenes. J. Am. Chem. Soc. 128, 694–695 (2006).
Acknowledgements
This work was supported by JST ERATO grant number JPMJER1302 (K.I.); JSPS KAKENHI grant numbers 19H05463 (K.I.), JP17H04868 (K.M.), JP19H02700 (K.M.), JP19H02701 (Y.S.) and JP19K22183 (Y.S.); Shorai Foundation for Science and Technology (K.M.); and the Noguchi Institute (Y.S. and K.M.). We thank Rigaku Co for assistance with the X-ray analysis. Calculations were performed using resources of the Research Center for Computational Science, Okazaki, Japan. ITbM is supported by the World Premier International Research Center Initiative (WPI), Japan.
Author information
Authors and Affiliations
Contributions
K.I. and K.M. conceived the concept and directed the project. Y.K. and S.M. conducted experiments. Y.S. performed X-ray crystallography. K.I and K.M. prepared the manuscript with feedback from the other authors.
Corresponding authors
Ethics declarations
Competing interests
K.I., K.M. and Y.K. are inventors on a patent application (JP 2019-091748) submitted by Nagoya University that covers the synthetic methods and its applications included in this paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Discussion, Tables 1–10, Figs. 1–16, and References.
Supplementary Data 1
Cartesian coordinates.
Supplementary Data
CIF file of compound 5d.
Supplementary Data
CIF file of compound 5e.
Supplementary Data
CIF file of compound 8a.
Rights and permissions
About this article
Cite this article
Matsubara, S., Koga, Y., Segawa, Y. et al. Creation of negatively curved polyaromatics enabled by annulative coupling that forms an eight-membered ring. Nat Catal 3, 710–718 (2020). https://doi.org/10.1038/s41929-020-0487-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-0487-0