Abstract
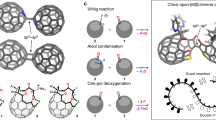
Mechanically interlocked carbon nanostructures represent a relatively unexplored frontier in carbon nanoscience due to the difficulty in preparing these unusual topological materials. Here we illustrate an active-template method in which a [n]cycloparaphenylene precursor macrocycle is decorated with two convergent pyridine donors that coordinate to a metal ion. The metal ion catalyses alkyne–alkyne cross-coupling reactions within the central cavity of the macrocycle, and the resultant interlocked products can be converted into fully π-conjugated structures in subsequent synthetic steps. Specifically, we report the synthesis of a family of catenanes that comprise two or three mutually interpenetrating [n]cycloparaphenylene-derived macrocycles of various sizes. Additionally, a fully π-conjugated [3]rotaxane was synthesized by the same method. The development of synthetic methods to access mechanically interlocked carbon nanostructures of varying topology can help elucidate the implications of mechanical bonding for this emerging class of nanomaterials and allow structure–property relationships to be established.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Experimental procedures for the synthesis of all the compounds are available in the Supplementary Information. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2159304 (1a), 2159303 (1b), 2159302 (2), 2159305 (diaza[8]CPP) and 2159306 (diaza[9]CPP). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. In addition to the spectra provided in the Supplementary Information, raw 1H and 13C data for all the novel structures are provided as Supplementary Data 6.
References
Thomas, S., Sarathchandran, C., Ilangovan, S. A. and Moreno-Pirajan, J. C. Handbook of Carbon-Based Nanomaterials (Elsevier, 2021).
Jasti, R., Bhattacharjee, J., Neaton, J. B. & Bertozzi, C. R. Synthesis, characterization, and theory of [9]-, [12]-, and [18]cycloparaphenylene: carbon nanohoop structures. J. Am. Chem. Soc. 130, 17646–17647 (2008).
Povie, G., Segawa, Y., Nishihara, T., Miyauchi, Y. & Itami, K. Synthesis of a carbon nanobelt. Science 356, 172–175 (2017).
Cheung, K. Y. et al. Synthesis of armchair and chiral carbon nanobelts. Chem 5, 838–847 (2019).
Cheung, K. Y., Watanabe, K., Segawa, Y. & Itami, K. Synthesis of a zigzag carbon nanobelt. Nat. Chem. 13, 255–259 (2021).
Darzi, E. R., Sisto, T. J. & Jasti, R. Selective syntheses of [7]–[12]cycloparaphenylenes using orthogonal Suzuki–Miyaura cross-coupling reactions. J. Org. Chem. 77, 6624–6628 (2012).
Lovell, T. C., Colwell, C. E., Zakharov, L. N. & Jasti, R. Symmetry breaking and the turn-on fluorescence of small, highly strained carbon nanohoops. Chem. Sci. 10, 3786–3790 (2019).
Hermann, M., Wassy, D. & Esser, B. Conjugated nanohoops incorporating donor, acceptor, hetero- or polycyclic aromatics. Angew. Chem. Int. Ed. 60, 15743–15766 (2021).
Leonhardt, E. J. & Jasti, R. Emerging applications of carbon nanohoops. Nat. Rev. Chem. 3, 672–686 (2019).
White, B. M. et al. Expanding the chemical space of biocompatible fluorophores: nanohoops in cells. ACS Cent. Sci. 4, 1173–1178 (2018).
Lovell, T. C. et al. Subcellular targeted nanohoop for one- and two-photon live cell imaging. ACS Nano 15, 15285–15293 (2021).
Peters, G. M. et al. Linear and radial conjugation in extended π-electron systems. J. Am. Chem. Soc. 142, 2293–2300 (2020).
Huang, Q. et al. A long π-conjugated poly(para-phenylene)-based polymeric segment of single-walled carbon nanotubes. J. Am. Chem. Soc. 141, 18938–18943 (2019).
Segawa, Y. et al. Topological molecular nanocarbons: all-benzene catenane and trefoil knot. Science 365, 272–276 (2019).
Aucagne, V., Hänni, K. D., Leigh, D. A., Lusby, P. J. & Walker, D. B. Catalytic ‘click’ rotaxanes: a substoichiometric metal-template pathway to mechanically interlocked architectures. J. Am. Chem. Soc. 128, 2186–2187 (2006).
Dietrich-Buchecker, C. O., Sauvage, J. P. & Kintzinger, J. P. Une nouvelle famille de molecules: les metallo-catenanes. Tetrahedron Lett. 24, 5095–5098 (1983).
Barin, G., Coskun, A., Fouda, M. M. G. & Stoddart, J. F. Mechanically interlocked molecules assembled by π–π recognition. ChemPlusChem. 77, 159–185 (2012).
Denis, M. & Goldup, S. M. The active template approach to interlocked molecules. Nat. Rev. Chem. 1, 0061 (2017).
Kubota, N., Segawa, Y. & Itami, K. η6-Cycloparaphenylene transition metal complexes: synthesis, structure, photophysical properties, and application to the selective monofunctionalization of cycloparaphenylenes. J. Am. Chem. Soc. 137, 1356–1361 (2015).
Van Raden, J. M., Louie, S., Zakharov, L. N. & Jasti, R. 2,2′-Bipyridyl-embedded cycloparaphenylenes as a general strategy to investigate nanohoop-based coordination complexes. J. Am. Chem. Soc. 139, 2936–2939 (2017).
Van Raden, J. M., White, B. M., Zakharov, L. N. & Jasti, R. Nanohoop rotaxanes from active metal template syntheses and their potential in sensing applications. Angew. Chem. Int. Ed. 58, 7341–7345 (2019).
Fan, Y. Y. et al. An isolable catenane consisting of two Möbius conjugated nanohoops. Nat. Commun. 9, 3037 (2018).
Berná, J. et al. Cadiot–Chodkiewicz active template synthesis of rotaxanes and switchable molecular shuttles with weak intercomponent interactions. Angew. Chem. Int. Ed. 47, 4392–4396 (2008).
Movsisyan, L. D. et al. Polyyne rotaxanes: stabilization by encapsulation. J. Am. Chem. Soc. 138, 1366–1376 (2016).
Hashimoto, S. et al. Synthesis and physical properties of polyfluorinated cycloparaphenylenes. Org. Lett. 20, 5973–5976 (2018).
Coropceanu, V. et al. Charge transport in organic semiconductors. Chem. Rev. 107, 926–952 (2007).
Slater, A. G. & Cooper, A. I. Function-led design of new porous materials. Science 348, aaa8075 (2015).
Darzi, E. R. & Jasti, R. The dynamic, size-dependent properties of [5]–[12]cycloparaphenylenes. Chem. Soc. Rev. 44, 6401–6410 (2015).
Acknowledgements
This project was supported by the National Science Foundation (CHE-1808791). J.H.M. and R.L.M. were additionally supported by National Science Foundation Graduate Research Fellowships.
Author information
Authors and Affiliations
Contributions
J.H.M., J.M.V.R. and R.J. were responsible for the conceptualization of the project. All the synthetic steps reported were performed by J.H.M. along with the computational and photophysical analyses. J.H.M. and R.J. drafted the original version of the manuscript with editing from J.M.V.R. and R.L.M. L.N.Z. collected and analysed all the crystallographic data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Birgit Esser and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental Details, NMR spectra of compounds, crystallographic information, computational details, Supplementary Figs. 1–72, discussion and Table 1.
Supplementary Data 1
.cif file for compound 1a.
Supplementary Data 2
.cif file for compound 1b..
Supplementary Data 3
.cif file for compound 2
Supplementary Data 4
.cif file for compound diaza[8]CPP.
Supplementary Data 5
.cif file for compound diaza[9]CPP.
Supplementary Data 6
.zip file containing 1H and 13 C NMR of all reported compounds.
Supplementary Data 7
.out file for the optimized structures of [11 + 2]CPP and [12 + 4]CPP.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
May, J.H., Van Raden, J.M., Maust, R.L. et al. Active template strategy for the preparation of π-conjugated interlocked nanocarbons. Nat. Chem. 15, 170–176 (2023). https://doi.org/10.1038/s41557-022-01106-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01106-9
This article is cited by
-
Palladium-catalyzed asymmetric carbene coupling en route to inherently chiral heptagon-containing polyarenes
Nature Communications (2024)
-
Interlocked structures on active duty
Nature Chemistry (2023)
-
A dodecamethoxy[6]cycloparaphenylene consisting entirely of hydroquinone ethers: unveiling in-plane aromaticity through a rotaxane structure
Nature Communications (2023)