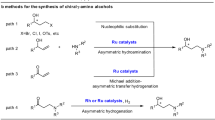
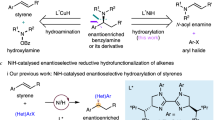
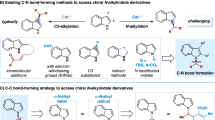
Abstract
Asymmetric hydrogenation of alkenes is one of the most powerful tools for the preparation of optically active compounds. However, to achieve high enantioselectivity, the starting olefin in most cases needs to be isomerically pure in either the cis or the trans form. Generally, most olefination protocols provide olefins as isomeric mixtures that are difficult to separate, and in many cases also generate lots of waste. In contrast, the synthesis of racemic alcohols is straightforward and highly atom-efficient, with products that are easier to purify. Here, we describe a strategy that enables rapid access to chiral alkanes via enantioconvergent formal deoxygenation of racemic alcohols. Mechanistic studies indicate an Ir-mediated elimination of water and subsequent in situ hydrogenation. This approach allows rapid and efficient assembly of chiral intermediates and is exemplified in the total synthesis of antidepressant sertraline and σ2 receptor PB 28.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data is available from the authors on reasonable request.
References
Roseblade, S. J. & Pfaltz, A. Iridium-catalyzed asymmetric hydrogenation of olefins. Acc. Chem. Res. 40, 1402–1411 (2007).
Chirik, P. J. Iron- and cobalt-catalyzed alkene hydrogenation: catalysis with both redox-active and strong field ligands. Acc. Chem. Res. 48, 1687–1695 (2015).
Verendel, J. J., Pàmies, O., Diéguez, M. & Andersson, P. G. Asymmetric hydrogenation of olefins using chiral crabtree-type catalysts: scope and limitations. Chem. Rev. 114, 2130–2169 (2014).
Zhu, S.-F. & Zhou, Q.-L. Iridium-catalyzed asymmetric hydrogenation of unsaturated carboxylic acids. Acc. Chem. Res. 50, 988–1001 (2017).
Minnaard, A. J., Feringa, B. L., Lefort, L. & de Vries, J. G. Asymmetric hydrogenation using monodentate phosphoramidite ligands. Acc. Chem. Res. 40, 1267–1277 (2007).
Zhang, W., Chi, Y. & Zhang, X. Developing chiral ligands for asymmetric hydrogenation. Acc. Chem. Res. 40, 1278–1290 (2007).
Etayoa, P. & Vidal-Ferran, A. Rhodium-catalysed asymmetric hydrogenation as a valuable synthetic tool for the preparation of chiral drugs. Chem. Soc. Rev. 42, 728–754 (2013).
Powell, M. T., Hou, D.-R., Perry, M. C., Cui, X. & Burgess, K. Chiral imidazolylidine ligands for asymmetric hydrogenation of aryl alkenes. J. Am. Chem. Soc. 123, 8878–8879 (2001).
Wang, A., Wstenberg, B. & Pfaltz, A. Enantio- and diastereoselective hydrogenation of farnesol and O-protected derivatives: stereocontrol by changing the C=C bond configuration. Angew. Chem. Int. Ed. 47, 2298–2300 (2008).
Peters, B. K. et al. An enantioselective approach to the preparation of chiral sulfones by Ir-catalyzed asymmetric hydrogenation. J. Am. Chem. Soc. 136, 16557–16562 (2014).
Liu, G., Han, Z., Dong, X.-Q. & Zhang, X. Rh-catalyzed asymmetric hydrogenation of β-substituted-β-thio-α,β-unsaturated esters: expeditious access to chiral organic sulfides. Org. Lett. 20, 5636–5639 (2018).
Yan, Q. et al. Highly efficient enantioselective synthesis of chiral sulfones by Rh-catalyzed asymmetric hydrogenation. J. Am. Chem. Soc. 141, 1749–1756 (2019).
Takaya, H. et al. Enantioselective hydrogenation of allylic and homoallylic alcohols. J. Am. Chem. Soc. 109, 1596–1597 (1987).
Maryanoff, B. E. & Reitz, A. B. The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. stereochemistry, mechanism, and selected synthetic aspects. Chem. Rev. 89, 863–927 (1989).
Thiemann, T. Wittig- and Horner–Wadsworth–Emmons olefination in aqueous media with and without phase transfer catalysis. Mini-Rev. Org. Chem. 15, 412–432 (2018).
Kita, Y. et al. Chloride-bridged dinuclear rhodium (III) complexes bearing chiral diphosphine ligands: catalyst precursors for asymmetric hydrogenation of simple olefins. Angew. Chem. Int. Ed. 55, 8299–8303 (2016).
Barton, D. H. R. & McCombie, S. W. A new method for the deoxygenation of secondary alcohols. J. Chem. Soc. 1, 1574–1585 (1975).
Nozaki, K., Oshima, K. & Utimoto, K. Facile reduction of dithiocarbonates with n-Bu3SnH-Et3B. Easy access to hydrocarbons from secondary alcohols. Tetrahedron Lett. 29, 6125–6126 (1988).
Lopez, R. M., Hays, D. S. & Fu, G. C. Bu3SnH catalyzed Barton−McCombie deoxygenation of alcohols. J. Am. Chem. Soc. 119, 6949–6950 (1997).
Drosos, N. & Morandi, B. Boron-catalyzed regioselective deoxygenation of terminal 1,2-diols to 2-alkanols enabled by the strategic formation of a cyclic siloxane intermediate. Angew. Chem. Int. Ed. 54, 8814–8818 (2015).
Foskey, T. J. A., Heinekey, D. M. & Goldberg, K. I. Partial deoxygenation of 1,2-propanediol catalyzed by iridium pincer complexes. ACS Catal. 2, 1285–1289 (2012).
Lao, D. B., Owens, A. C. E., Heinekey, D. M. & Goldberg, K. I. Partial deoxygenation of glycerol catalyzed by iridium pincer complexes. ACS Catal. 3, 2391–2396 (2013).
Schlaf, M., Ghosh, P., Fagan, P. J., Hauptman, E. & Bullock, R. M. Metal-catalyzed selective deoxygenation of diols to alcohols. Angew. Chem. Int. Ed. 40, 3887–3890 (2001).
Schlaf, M., Ghosh, P., Fagan, P. J., Hauptman, E. & Bullock, R. M. Catalytic deoxygenation of 1,2-propanediol to give n-propanol. Adv. Synth. Catal. 351, 789–800 (2009).
Dai, X.-J. & Li, C.-J. En route to a practical primary alcohol deoxygenation. J. Am. Chem. Soc. 138, 5433–5440 (2016).
Huang, J.-L., Dai, X.-J. & Li, C.-J. Iridium-catalyzed direct dehydroxylation of alcohols. Eur. J. Org. Chem. 2013, 6496–6500 (2013).
Bauer, J. O., Chakraborty, S. & Milstein, D. Manganese-catalyzed direct deoxygenation of primary alcohols. ACS Catal. 7, 4462–4466 (2017).
Yang, S., Tang, W., Yang, Z. & Xu, J. Iridium-catalyzed highly efficient and site-selective deoxygenation of alcohols. ACS Catal. 8, 9320–9326 (2018).
Isomura, M., Petrone, D. A. & Carreira, E. M. Coordination-induced stereocontrol over carbocations: asymmetric reductive deoxygenation of racemic tertiary alcohols. J. Am. Chem. Soc. 141, 4738–4748 (2019).
Liu, J., Krajangsri, S., Yang, J., Li, J.-Q. & Andersson, P. G. Iridium-catalysed asymmetric hydrogenation of allylic alcohols via dynamic kinetic resolution. Nat. Catal. 1, 438–443 (2018).
Krajangsri, S. et al. Tandem Peterson olefination and chemoselective asymmetric hydrogenation of β-hydroxy silanes. Chem. Sci. 10, 3649–3653 (2019).
Kerdphon, S. et al. Diastereo- and enantioselective synthesis of structurally diverse succinate, butyrolactone, and trifluoromethyl derivatives by iridium-catalyzed hydrogenation of tetrasubstituted olefins. ACS Catal. 9, 6169–6176 (2019).
Poremba, K. E., Kadunce, N. T., Suzuki, N., Cherney, A. H. & Reisman, S. E. Nickel-catalyzed asymmetric reductive cross-coupling to access 1,1-diarylalkanes. J. Am. Chem. Soc. 139, 5684–5687 (2017).
Berardi, F. et al. 4-(Tetralin-1-yl)- and 4-(naphthalen-1-yl)alkyl derivatives of 1-cyclohexylpiperazine as σ receptor ligands with agonist σ 2 activity. J. Med. Chem. 47, 2308–2317 (2004).
Church, T. L., Rasmussen, T. & Andersson, P. G. Enantioselectivity in the iridium catalyzed hydrogenation of unfunctionalized olefins. Organometallics 29, 6769–6781 (2010).
Acknowledgements
The Swedish Research Council (VR), Stiftelsen Olle Engkvist Byggmästare, the Carl Tryggers Stiftelse, Knut and Alice Wallenberg foundation (KAW 2016.0072 and KAW 2018:0066) supported this work. J.J. thanks the Scientist Development Project Commemorating His Majesty the King’s 84th Birthday Anniversary, Chulabhorn Graduate Institute. We are also thankful to D.A. Tanner and M. Nolan for proofreading the manuscript.
Author information
Authors and Affiliations
Contributions
P.G.A. conceived and designed the experiments. J.Z., J.J., B.B.C.P., J.G. and S.P. performed experiments and prepared the Supplementary Information. M.S.G.A. performed the computational studies. P.G.A. and J.Z. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5, Tables 1–6, discussion, methods and references.
Supplementary Data 1
Cartesian coordinates of optimized structures.
Rights and permissions
About this article
Cite this article
Zheng, J., Jongcharoenkamol, J., Peters, B.B.C. et al. Iridium-catalysed enantioselective formal deoxygenation of racemic alcohols via asymmetric hydrogenation. Nat Catal 2, 1093–1100 (2019). https://doi.org/10.1038/s41929-019-0375-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0375-7