Abstract
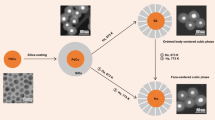
Bimetallic and multi-component catalysts typically exhibit composition-dependent activity and selectivity, and when optimized often outperform single-component catalysts. Here we used ambient-pressure X-ray photoelectron spectroscopy (AP-XPS) and in situ and ex situ transmission electron microscopy (TEM) to elucidate the origin of composition dependence observed in the catalytic activities of monodisperse CoPd bimetallic nanocatalysts for CO oxidation. We found that the catalysis process induced a reconstruction of the catalysts, leaving CoOx on the nanoparticle surface. The synergy between Pd and CoOx coexisting on the surface promotes the catalytic activity of the bimetallic catalysts. This synergistic effect can be optimized by tuning the Co/Pd ratios in the nanoparticle synthesis, and it reaches a maximum at compositions near Co0.24Pd0.76, which achieves complete CO conversion at the lowest temperature. Our combined AP-XPS and TEM studies provide direct observation of the surface evolution of the bimetallic nanoparticles under catalytic conditions and show how this evolution correlates with catalytic properties.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
Change history
21 January 2019
In the version of this Article originally published, the author Baran Eren was mistakenly affiliated with the Harbin Institute of Technology, China; it has now been corrected to Materials Science Division, Lawrence Berkeley National Laboratory, Berkeley, CA, USA.
References
Dyakonov, A. & Robinson, E. Low-temperature oxidation of CO in smoke: a review. Beitr. Tabakforsch. 22, 88–106 (2006).
van Spronsen, M. A., Frenken, J. W. M. & Groot, I. M. N. Surface science under reaction conditions: CO oxidation on Pt and Pd model catalysts. Chem. Soc. Rev. 46, 4347–4374 (2017).
Imbihl, R. Oscillatory reactions on single crystal surfaces. Prog. Surf. Sci. 44, 185–343 (1993).
Rodriguez, J. A. & Goodman, D. W. High-pressure catalytic reactions over single-crystal metal-surfaces. Surf. Sci. Rep. 14, 1–107 (1991).
Freund, H. J., Meijer, G., Scheffler, M., Schlögl, R. & Wolf, M. CO oxidation as a prototypical reaction for heterogeneous processes. Angew. Chem. Int. Ed. 50, 10064–10094 (2011).
Heck, R. M. & Farrauto, R. J. Automobile exhaust catalysts. Appl. Catal. A 221, 443–457 (2001).
Twigg, M. V. Progress and future challenges in controlling automotive exhaust gas emissions. Appl. Catal. B 70, 2–15 (2007).
Nilekar, A. U., Alayoglu, S., Eichhorn, B. & Mavrikakis, M. Preferential CO oxidation in hydrogen: reactivity of core–shell nanoparticles. J. Am. Chem. Soc. 132, 7418–7428 (2010).
Fu, Q. et al. Interface-confined ferrous centers for catalytic oxidation. Science 328, 1141–1144 (2010).
Saavedra, J., Doan, Ha, Pursell, C. J., Grabow, L. C. & Chandler, B. D. The critical role of water at the gold-titania interface in catalytic CO oxidation. Science 345, 1599–1602 (2014).
Wang, Y. G., Yoon, Y., Glezakou, V. A., Li, J. & Rousseau, R. The role of reducible oxide-metal cluster charge transfer in catalytic processes: new insights on the catalytic mechanism of CO oxidation on Au/TiO2 from ab initio molecular dynamics. J. Am. Chem. Soc. 135, 10673–10683 (2013).
Kim, H. Y., Lee, H. M. & Henkelman, G. CO oxidation mechanism on CeO2-supported Au nanoparticles. J. Am. Chem. Soc. 134, 1560–1570 (2012).
Widmann, D. & Behm, R. J. Active oxygen on a Au/TiO2 catalyst: formation, stability, and CO oxidation activity. Angew. Chem. Int. Ed. 50, 10241–10245 (2011).
Haruta, M., Kobayashi, T., Sano, H. & Yamada, N. Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0 °C. Chem. Lett. 16, 405–408 (1987).
Wang, C., Yin, H., Dai, S. & Sun, S. A general approach to noble metal−metal oxide dumbbell nanoparticles and their catalytic application for CO oxidation. Chem. Mater. 22, 3277–3282 (2010).
An, K. et al. Enhanced CO oxidation rates at the interface of mesoporous oxides and Pt nanoparticles. J. Am. Chem. Soc. 135, 16689–16696 (2013).
Cargnello, M. et al. Control of metal nanocrystal size reveals metal-support interface role for ceria catalysts. Science 341, 771–773 (2013).
Zhu, H. et al. Constructing hierarchical interfaces: TiO2-supported PtFe–FeOx nanowires for room temperature CO oxidation. J. Am. Chem. Soc. 137, 10156–10159 (2015).
Chen, G. et al. Interfacial effects in iron–nickel hydroxide–platinum nanoparticles enhance catalytic oxidation. Science 344, 495–499 (2014).
Shan, S. et al. Atomic-structural synergy for catalytic CO oxidation over palladium-nickel nanoalloys. J. Am. Chem. Soc. 136, 7140–7151 (2014).
Zhan, W. et al. Crystal structural effect of AuCu alloy nanoparticles on catalytic CO oxidation. J. Am. Chem. Soc. 139, 8846–8854 (2017).
Gilroy, K. D., Ruditskiy, A., Peng, H.-C., Qin, D. & Xia, Y. Bimetallic nanocrystals: syntheses, properties, and applications. Chem. Rev. 116, 10414–10472 (2016).
Yu, W., Porosoff, M. D. & Chen, J. G. Review of Pt-based bimetallic catalysis: from model surfaces to supported catalysts. Chem. Rev. 112, 5780–5817 (2012).
Guo, S., Zhang, S. & Sun, S. Tuning nanoparticle catalysis for the oxygen reduction reaction. Angew. Chem. Int. Ed. 52, 8526–8544 (2013).
Tao, F. et al. Reaction-driven restructuring of Rh–Pd and Pt–Pd core–shell nanoparticles. Science 322, 932–934 (2008).
Tao, F. et al. Evolution of structure and chemistry of bimetallic nanoparticle catalysts under reaction conditions. J. Am. Chem. Soc. 132, 8697–8703 (2010).
Carenco, S. et al. Dealloying of cobalt from CuCo nanoparticles under syngas exposure. J. Phys. Chem. C 117, 6259–6266 (2013).
Ogletree, D. F. et al. A differentially pumped electrostatic lens system for photoemission studies in the millibar range. Rev. Sci. Instrum. 73, 3872 (2002).
Wu, C. H., Weatherup, R. S. & Salmeron, M. B. Probing electrode/electrolyte interfaces in situ by X-ray spectroscopies: old methods, new tricks. Phys. Chem. Chem. Phys. 17, 30229–30239 (2015).
Zheng, H., Meng, Y. S. & Zhu, Y. Frontiers of in situ electron microscopy. MRS Bull. 40, 12–18 (2015).
Escudero, C. et al. A reaction cell with sample laser heating for in situ soft X-ray absorption spectroscopy studies under environmental conditions. J. Synch. Radiat. 20, 504–508 (2013).
Heine, C. et al. Ambient-pressure soft X-ray absorption spectroscopy of a catalyst surface in action: closing the pressure gap in the selective n-butane oxidation over vanadyl pyrophosphate. J. Phys. Chem. C 118, 20405–20412 (2014).
Bluhm, H., Ogletree, D. F., Fadley, C. S., Hussain, Z. & Salmeron, M. The premelting of ice studied with photoelectron spectroscopy. J. Phys. Condens. Matter. 14, L227–L233 (2002).
Sun, D., Mazumder, V., Metin, O. & Sun, S. Catalytic hydrolysis of ammonia borane via cobalt palladium nanoparticles. ACS Nano 5, 6458–6464 (2011).
Sun, S. & Murray, C. B. Synthesis of monodisperse cobalt nanocrystals and their assembly into magnetic superlattices (invited). J. Appl. Phys. 85, 4325–4330 (1999).
Zhang, S. et al. Monodisperse core/shell Ni/FePt nanoparticles and their conversion to Ni/Pt to catalyze oxygen reduction. J. Am. Chem. Soc. 136, 15921–15924 (2014).
Ishida, K. & Nishizawa, T. The C–Co (carbon–cobalt) system. J. Phase Equilibria 12, 417–424 (1991).
Toyoshima, R. et al. Active surface oxygen for catalytic CO oxidation on Pd(100) proceeding under near ambient pressure conditions. J. Phys. Chem. Lett. 3, 3182–3187 (2012).
Ketteler, G. et al. In situ spectroscopic study of the oxidation and reduction of Pd(111). J. Am. Chem. Soc. 127, 18269–18273 (2005).
Gries, W. H. Angular intensity modulation in angle-resolved XPS and AES of non-crystalline ultrathin surface layers: the phenomenon and its implications. Surf. Interface Anal. 17, 803–812 (1991).
Kibis, L. S., Titkov, A. I., Stadnichenko, A. I., Koscheev, S. V. & Boronin, A. I. X-ray photoelectron spectroscopy study of Pd oxidation by RF discharge in oxygen. Appl. Surf. Sci. 255, 9248–9254 (2009).
Militello, M. C. Palladium oxide (PdO) by XPS. Surf. Sci. Spectra 3, 395 (1994).
Balmes, O. et al. Reversible formation of a PdCx phase in Pd nanoparticles upon CO and O2 exposure. Phys. Chem. Chem. Phys. 14, 4796 (2012).
Rogal, J., Reuter, K. & Scheffler, M. Oxidation at Pd(100): a first-principles constrained thermodynamics study. Phys. Rev. B 75, 1–11 (2007).
Wu, C. H., Eren, B., Bluhm, H. & Salmeron, M. B. Ambient-pressure X-ray photoelectron spectroscopy study of cobalt foil model catalyst under CO, H2, and their mixtures. ACS Catal. 7, 1150–1157 (2017).
Wang, Z. L., Bentley, J. & Evans, N. D. Valence state mapping of cobalt and manganese using near-edge fine structures. Micron 31, 355–362 (2000).
Wang, Z. L., Yin, J. S. & Jiang, Y. D. EELS analysis of cation valence states and oxygen vacancies in magnetic oxides. Micron 31, 571–580 (2000).
Lee, A. F. & Lambert, R. M. Oxidation of Sn overlayers and the structure and stability of Sn oxide films on Pd(111). Phys. Rev. B 58, 4156–4165 (1998).
Batzill, M., Beck, D. E., Jerdev, D. & Koel, B. E. Tin-oxide overlayer formation by oxidation of Pt–Sn(111) surface alloys. J. Vac. Sci. Technol. A 19, 1953 (2001).
Acknowledgements
This work was supported by the Office of Basic Energy Sciences of the US Department of Energy under contract no. DE-AC02-05CH11231 through the Chemical Sciences, Geosciences, and Biosciences Division. Funding from the same contract for the ALS and beamline 9.3.2 is also acknowledged. Partial work on CoPd nanoparticles synthesis and characterization were supported by US National Science Foundation (DMR-1809700) and Jeffress Trust Awards Program in Interdisciplinary Research from Thomas F. and Kate Miller Jeffress Memorial Trust. Partial work on electron microscopy carried out at the Center for Functional Nanomaterials, Brookhaven National Laboratory, was supported by the US Department of Energy, Office of Basic Energy Sciences, under contract no. DE-AC02-98CH10886.
Author information
Authors and Affiliations
Contributions
The project was conceived by C.H.W. and C.L. under the supervision of S.Z, M.B.S. and C.B.M. Catalyst synthesis, basic characterization and catalytic activity measurements were performed by C.L. and S.Z. AP-XPS experiments were conducted by C.H.W., H.-T.F. and B.E. Ex situ TEM and elemental mapping were done by D.S. In situ TEM and EELS measurements were performed by H.X., C.H.W. and S.Z. Ex situ XAS measurements were done by C.H.W. The analysis and interpretation of all spectra (XPS, XAS and EELS) were done by C.H.W. All authors contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figures 1–12.
Rights and permissions
About this article
Cite this article
Wu, C.H., Liu, C., Su, D. et al. Bimetallic synergy in cobalt–palladium nanocatalysts for CO oxidation. Nat Catal 2, 78–85 (2019). https://doi.org/10.1038/s41929-018-0190-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0190-6
This article is cited by
-
Metastable gallium hydride mediates propane dehydrogenation on H2 co-feeding
Nature Chemistry (2024)
-
Advances in in situ/operando techniques for catalysis research: enhancing insights and discoveries
Surface Science and Technology (2024)
-
Highly active copper-intercalated weakly crystallized δ-MnO2 for low-temperature oxidation of CO in dry and humid air
Frontiers of Environmental Science & Engineering (2024)
-
Atomically dispersed materials: Ideal catalysts in atomic era
Nano Research (2024)
-
Elucidating the active phases of CoOx films on Au(111) in the CO oxidation reaction
Nature Communications (2023)