Abstract
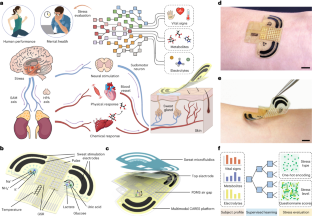
Approaches to quantify stress responses typically rely on subjective surveys and questionnaires. Wearable sensors can potentially be used to continuously monitor stress-relevant biomarkers. However, the biological stress response is spread across the nervous, endocrine and immune systems, and the capabilities of current sensors are not sufficient for condition-specific stress response evaluation. Here we report an electronic skin for stress response assessment that non-invasively monitors three vital signs (pulse waveform, galvanic skin response and skin temperature) and six molecular biomarkers in human sweat (glucose, lactate, uric acid, sodium ions, potassium ions and ammonium). We develop a general approach to prepare electrochemical sensors that relies on analogous composite materials for stabilizing and conserving sensor interfaces. The resulting sensors offer long-term sweat biomarker analysis of more than 100 h with high stability. We show that the electronic skin can provide continuous multimodal physicochemical monitoring over a 24-hour period and during different daily activities. With the help of a machine learning pipeline, we also show that the platform can differentiate three stressors with an accuracy of 98.0% and quantify psychological stress responses with a confidence level of 98.7%.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The multimodal data collected by the CARES from human subjects is available at https://github.com/CARES-eskin/StressData. All other data that support the findings of this study are available from the corresponding author on reasonable request.
References
Kivimäki, M., Bartolomucci, A. & Kawachi, I. The multiple roles of life stress in metabolic disorders. Nat. Rev. Endocrinol. 19, 10–27 (2022).
Schneiderman, N., Ironson, G. & Siegel, S. D. Stress and health: psychological, behavioral, and biological determinants. Annu. Rev. Clin. Psychol. 1, 607–628 (2005).
Kumar, A., Rinwa, P., Kaur, G. & Machawal, L. Stress: neurobiology, consequences and management. J. Pharm. Bioallied Sci. 5, 91–97 (2013).
Podsakoff, N. P., Freiburger, K. J., Podsakoff, P. M. & Rosen, C. C. Laying the foundation for the challenge–hindrance stressor framework 2.0. Annu. Rev. Organ. Psychol. Organ. Behav. 10, 165–199 (2023).
Pfefferbaum, B. & North, C. S. Mental health and the COVID-19 pandemic. N. Engl. J. Med. 383, 510–512 (2020).
Santomauro, D. F. et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 398, 1700–1712 (2021).
Gutshall, C. L., Hampton, D. P., Sebetan, I. M., Stein, P. C. & Broxtermann, T. J. The effects of occupational stress on cognitive performance in police officers. Police Pract. Res. 18, 463–477 (2017).
Tomporowski, P. D. Effects of acute bouts of exercise on cognition. Acta Psychol. 112, 297–324 (2003).
Martin, K. et al. The impact of environmental stress on cognitive performance: a systematic review. Hum. Factors 61, 1205–1246 (2019).
Robinson, S. J., Leach, J., Owen-Lynch, P. J. & Sünram-Lea, S. I. Stress reactivity and cognitive performance in a simulated firefighting emergency. Aviat. Space Environ. Med. 84, 592–599 (2013).
Haines, M. M., Stansfeld, S. A., Job, R. F. S., Berglund, B. & Head, J. Chronic aircraft noise exposure, stress responses, mental health and cognitive performance in school children. Psychol. Med. 31, 265–277 (2001).
Kulshreshtha, A. et al. Association of stress with cognitive function among older black and white US adults. JAMA Netw. Open 6, e231860 (2023).
Epel, E. S. et al. More than a feeling: a unified view of stress measurement for population science. Front. Neuroendocrinol. 49, 146–169 (2018).
Thapar, A., Eyre, O., Patel, V. & Brent, D. Depression in young people. Lancet 400, 617–631 (2022).
Topol, E. Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again (Basic Books, 2019).
Herrman, H. et al. Time for united action on depression: a Lancet–World Psychiatric Association Commission. Lancet 399, 957–1022 (2022).
Drew, D. A. et al. Rapid implementation of mobile technology for real-time epidemiology of COVID-19. Science 368, 1362–1367 (2020).
Charmandari, E., Tsigos, C. & Chrousos, G. Endocrinology of the stress response. Annu. Rev. Physiol. 67, 259–284 (2005).
Dolan, R. J. Emotion, cognition, and behavior. Science 298, 1191–1194 (2002).
Harker, M. Psychological sweating: a systematic review focused on aetiology and cutaneous response. Skin Pharmacol. Physiol. 26, 92–100 (2013).
Axelrod, J. & Reisine, T. D. Stress hormones: their interaction and regulation. Science 224, 452–459 (1984).
Acosta, J. N., Falcone, G. J., Rajpurkar, P. & Topol, E. J. Multimodal biomedical AI. Nat. Med. 28, 1773–1784 (2022).
Buergel, T. et al. Metabolomic profiles predict individual multidisease outcomes. Nat. Med. 28, 2309–2320 (2022).
Ates, H. C. et al. End-to-end design of wearable sensors. Nat. Rev. Mater. 7, 887–907 (2022).
Wang, C. et al. Bioadhesive ultrasound for long-term continuous imaging of diverse organs. Science 377, 517–523 (2022).
Kim, D.-H. et al. Epidermal electronics. Science 333, 838–843 (2011).
Xu, C., Yang, Y. & Gao, W. Skin-interfaced sensors in digital medicine: from materials to applications. Matter 2, 1414–1445 (2020).
Niu, S. et al. A wireless body area sensor network based on stretchable passive tags. Nat. Electron. 2, 361–368 (2019).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Wang, M. et al. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 6, 1225–1235 (2022).
Kim, J., Campbell, A. S., de Ávila, B. E.-F. & Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 37, 389–406 (2019).
Ray, T. R. et al. Bio-integrated wearable systems: a comprehensive review. Chem. Rev. 119, 5461–5533 (2019).
Chesnut, M. et al. Stress markers for mental states and biotypes of depression and anxiety: a scoping review and preliminary illustrative analysis. Chronic Stress 5, 24705470211000338 (2021).
Xu, S., Kim, J., Walter, J. R., Ghaffari, R. & Rogers, J. A. Translational gaps and opportunities for medical wearables in digital health. Sci. Transl. Med. 14, eabn6036 (2022).
Torrente-Rodríguez, R. M. et al. Investigation of cortisol dynamics in human sweat using a graphene-based wireless mHealth system. Matter 2, 921–937 (2020).
Wang, B. et al. Wearable aptamer-field-effect transistor sensing system for noninvasive cortisol monitoring. Sci. Adv. 8, eabk0967 (2022).
Sheibani, S. et al. Extended gate field-effect-transistor for sensing cortisol stress hormone. Commun. Mater. 2, 10 (2021).
Simmers, P., Li, S. K., Kasting, G. & Heikenfeld, J. Prolonged and localized sweat stimulation by iontophoretic delivery of the slowly-metabolized cholinergic agent carbachol. J. Dermatol. Sci. 89, 40–51 (2018).
Sancini, A. & Tomei, F. Work related stress and blood glucose levels. Ann. Ig. 29, 123–133 (2017).
Hermann, R., Lay, D., Wahl, P., Roth, W. T. & Petrowski, K. Effects of psychosocial and physical stress on lactate and anxiety levels. Stress 22, 664–669 (2019).
Kubera, B. et al. Rise in plasma lactate concentrations with psychosocial stress: a possible sign of cerebral energy demand. Obes. Facts 5, 384–392 (2012).
Klous, L., de Ruiter, C. J., Scherrer, S., Gerrett, N. & Daanen, H. A. M. The (in)dependency of blood and sweat sodium, chloride, potassium, ammonia, lactate and glucose concentrations during submaximal exercise. Eur. J. Appl. Physiol. 121, 803–816 (2021).
Goodman, A. M. et al. The hippocampal response to psychosocial stress varies with salivary uric acid level. Neuroscience 339, 396–401 (2016).
Nyein, H. Y. Y. et al. A wearable electrochemical platform for noninvasive simultaneous monitoring of Ca2+ and pH. ACS Nano 10, 7216–7224 (2016).
Lin, S. et al. Wearable microneedle-based electrochemical aptamer biosensing for precision dosing of drugs with narrow therapeutic windows. Sci. Adv. 8, eabq4539 (2022).
Tu, J. et al. A wireless patch for the monitoring of C-reactive protein in sweat. Nat. Biomed. Eng. 7, 1293–1306 (2023).
Shao, Y., Ying, Y. & Ping, J. Recent advances in solid-contact ion-selective electrodes: functional materials, transduction mechanisms, and development trends. Chem. Soc. Rev. 49, 4405–4465 (2020).
Spielberger, C. D., Gorsuch, R. L., Lushene, R. E., Vagg, P. R. & Jacobs, G. A. (eds) Manual for the State-trait Anxiety Inventory (STAI Form Y) (Consulting Psychologists Press, 1983).
Frank, S. M. & Raja, S. N. Reflex cutaneous vasoconstriction during cold pressor test is mediated through α-adrenoceptors. Clin. Auton. Res. 4, 257–261 (1994).
Schwabe, L., Haddad, L. & Schachinger, H. HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology 33, 890–895 (2008).
Khambam, S. K. R., Naidu, M. U. R., Rani, P. U. & Rao, T. R. K. Effect of cold stimulation-induced pain on pharmacodynamic responses in healthy human volunteers. Int. J. Nutr. Pharmacol. Neurol. Dis. 2, 26 (2012).
Buono, M. J., Lee, N. V. L. & Miller, P. W. The relationship between exercise intensity and the sweat lactate excretion rate. J. Physiol. Sci. 60, 103–107 (2010).
Maaten van der, L. & Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 9, 2579–2605 (2008).
Hay, E. L. & Diehl, M. Reactivity to daily stressors in adulthood: the importance of stressor type in characterizing risk factors. Psychol. Aging 25, 118–131 (2010).
Crestani, C. C. Emotional stress and cardiovascular complications in animal models: a review of the influence of stress type. Front. Physiol. 7, 251 (2016).
Pow, J., Lee-Baggley, D. & DeLongis, A. Threats to communion and agency mediate associations between stressor type and daily coping. Anxiety Stress Coping 29, 660–672 (2016).
Scheid, T. L. & Brown, T. N. (eds) Handbook for the Study of Mental Health: Social Contexts, Theories, and Systems. (Cambridge Univ. Press, 2009); https://doi.org/10.1017/CBO9780511984945
Acknowledgements
This work was funded by the Translational Research Institute for Space Health through NASA NNX16AO69A, Office of Naval Research grant nos. N00014-21-1-2483 and N00014-21-1-2845, Army Research Office grant no. W911NF-23-1-0041, National Institutes of Health grant nos. R01HL155815 and R21DK13266, National Science Foundation grant no. 2145802, National Academy of Medicine Catalyst Award and High Impact Pilot Research Award no. T31IP1666 from the Tobacco-Related Disease Research Program and Heritage Medical Research Institute (all to W.G.). T.K.H. acknowledges the support from National Institutes of Health grant nos. T32HL144449 and T32EB027629. C.X. acknowledges support from an Amazon AI4Science Fellowship. ICP-MS instrumentation at the Resnick Sustainability Institute’s Water and Environment Lab at the California Institute of Technology was used in this work with the assistance of N. Dalleska. We acknowledge critical support and infrastructure provided for this work by the Kavli Nanoscience Institute at Caltech and Center for Transmission Electron Microscopy at the University of California Irvine, and we thank M. Hunt and M. Xu for their help.
Author information
Authors and Affiliations
Contributions
W.G. and C.X. conceived the project. C.X. led the sensors and CARES platform development. C.X., Y.S. and J.R.S. led the platform characterization and human studies. S.A.S. and J.L. contributed to the data processing and feature extraction. H.Y.Y.N. contributed to sensor development. Y.Y., R.Y.T. and A.L. contributed to sensor characterization and testing. W.H. and J.M. contributed to wireless system development. T.K.H. and J.A.S. contributed to the human study design. W.G., C.X., Y.S., J.R.S. and S.A.S. cowrote the paper. All authors contributed to the data analysis and provided feedback on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Electronics thanks Sihong Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–9, Tables 1–4, Figs. 1–48 and Videos 1 and 2.
Supplementary Video 1
The two-reservoir microfluidic module during an IP-induced sweat secretion process.
Supplementary Video 2
Multiplexed and multimodal data collection with real-life activities using the fully integrated wireless CARES patch.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, C., Song, Y., Sempionatto, J.R. et al. A physicochemical-sensing electronic skin for stress response monitoring. Nat Electron 7, 168–179 (2024). https://doi.org/10.1038/s41928-023-01116-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41928-023-01116-6
This article is cited by
-
Stress monitoring with wearable technology and AI
Nature Electronics (2024)
-
Connecting the work of scientists, engineers and industry
Nature Electronics (2024)